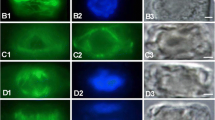
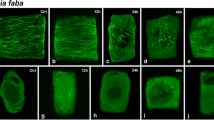
Abstract
Caffeine, (1:3:7-tri-methyl-xanthine), either as a prefixation treatment or included with glutaralde-hyde as the primary fixative, destroys or disorganises the microtubules associated with the formation of secondary walls in fibres from the flowering stem of the grass Lolium temulentum L. There is no observable effect of caffeine treatment on the microtubules associated with primary wall formation in collenchyma and young fibres from L. temulentum or in root cap cells of Zea mays L. and Phaseolus vulgaris L. The microtubules associated with primary wall formation are destroyed by cold treatment but not those associated with secondary wall formation. Tannic acid included in the fixative shows the microtubules associated with secondary wall formation in fibres of L. temulentum to be composed of 13 subunits. Treatment with lanthanum hydroxide does not stain the core or the halo of the microtubules.
Similar content being viewed by others
Abbreviations
- PIPES:
-
Piperazine N-N- bis 2 ethanol sulphonic acid
References
Anderson, J.W.: Extraction of enzymes and subcellular organelles from plant tissue. Phytochemistry 7, 1973–1988 (1968)
Bajer, A.S., Molè-Bajer, J.: Spindle dynamics and chromosome movements. Int. Rev. Cytol. Suppl. 3 (1972)
Behnke, O.: A comparative study of microtubules of disk shaped blood cells. J. Ultrastruct. Res. 31, 61–75 (1970)
Behnke, O., Forer, A.: Evidence for 4 classes of microtubules in individual cells. J. Cell. Sci. 2, 169–192 (1967)
Burton, P.R., Fernandez, H.L.: Delineation by lanthanum staining of filamentous elements associated with the surfaces of axonal microtubules. J. Cell Sci. 12, 567–583 (1973)
Chen, M.H., Hiruki, C.: the preservation of membranes of tubular bodies associated with mycoplasmalike organisms by tannic acid. Can. J. Bot. 56, 2878–2882 (1978)
Cox, G.C.: The structure and development of cells with thickened primary walls. Oxford: Thesis 1971
Doggenweiler, C.F., Frenk, S.: Staining properties of lanthanum on cell membranes. Proc. Nat. Acad. Sci. USA 53, 425–430 (1965)
Gunning, B.E.S., Steer, M.W.: Ultrastructure and the biology of plant cells. London: Arnold 1975
Harris, P.J., Hartley, R.D.: The detection of bound ferulic acid in cell walls of Gramineae by U.V. fluorescence microscopy. Nature (London) 259, 508–510 (1976)
Hepler, P.K.: Plant Microtubules. In: Plant Biochemistry, 3rd. Bonner, J., Varner, J.E. eds. New York: Academic Press 1976
Hepler, P.K., Palevitz, B.A.: Microtubules and microfilaments. Ann. Rev. Plant Physiol. 25, 309–362 (1974)
Hinkley, R.E.: Microtubule-Macrotubule transformation induced by volatile anaesthetics: Mechanism of macrotubule assembly. J. Ultrastruct. Res. 57, 237–252 (1976)
Juniper, B.E., Cox, G.C., Gilchrist, A.J., Williams, P.R.: Techniques for plant electron microscopy. Oxford, Edinburgh: Blackwell 1970
Lane, N.J., Treherne, J.E.: Lanthanum staining of neurotubules in axons from cockroach ganglia. J. Cell Sci. 7, 217–231 (1970)
Lawton, R., Harris, P.J.: Fixation of senescing plant tissues: sclerenchymatous fibre cells from the flowering stem of a grass. J. Microsc. (in press).
Lawton, J.R., Harris, P.J., Juniper, B.E.: Ultrastructural aspects of the development of fibres from the flowering stem of Lolium temulentum L. New Phytol. (in press)
Ledbetter, M.C., Porter, K.R.: A “microtubule” in plant cell fine structure. J. Cell Biol. 19, 239–250 (1963)
Letbetter, M.C., Porter, K.R.: Morphology of microtubules of plant cells. Science 144, 872–874 (1964)
Mejbaum-Katzenellenbogen, W., Dobryszycka, W., Boguslawska-Jaworska, J., Morawiecka, B.: Regeneration of protein from insoluble protein-tannin compounds. Nature London 184, 1799–1800 (1959)
Mollenhauer, H.H.: Plastic embedding mixtures for use in electron microscopy. Stein Technol. 39, 111–114 (1964)
Mueller, W.C., Rodenhurst, E.: The effect of some alkaloids on the ultrastructure of phenolic-containing cells in the endodermis of cotton roots 35th, Bailey, G.W. ed. Ann. Proc. Electron Microscopy Soc. Amer. Boston, Mass. 1977
Nelmes, B.J., Preston, R.D., Ashworth, D.: A possible function of microtubules suggested by their distribution in rubbery wood. J. Cell Sci. 13, 741–751 (1973)
Pickett-Heaps, J.D.: The effects of colchicine on the ultrastructure of dividing cells. Xylem wall differentiation and distribution of cytoplasmic microtubules. Develop. Biol. 15, 206–236 (1967)
Pickett-Heaps, J.D.: Preprophase microtubules in some abnormal cells of wheat. J. Cell Sci. 4, 397–420 (1969)
Revel, J.P., Karnovsky, M.J.: Hexagonal array of subunits in intracellular junctions of the mouse heart and liver. J. Cell Biol. 33, C7-C12. (1967)
Reynolds, E.S.: The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963)
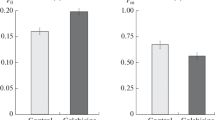
Röper, W., Röper, S.: Centripetal wall formation in roots of Vicia faba after caffeine treatment. Protoplasma 93, 89–100 (1977)
Roth, L.E., Shigenaka, Y.: Microtubules in the Heliozoan axopodium. Rapid degradation by cupric and nickelous ions. J. Ultrastruct. Res. 31, 356–371 (1970)
Roth, L.E., Pihlaja, D.J., Shigenaka, Y.: Microtubules in the heliozoan axopodium 1. The gradion hypothesis of allosterism in structural proteins. J. Ultrastruct. Res. 30, 7–37 (1970)
Spurr, A.R.: A low-viscosity embedding resin medium for electron microscopy. J. Ultrastruct. Res. 26, 31 (1969)
Tilney, L.G., Bryan, J., Bush, D.J., Fujiwara, K., Mooseker, M.S., Murphy, D.B., Snyder, D.H.: Microtubules: evidence for 13 protofilaments. J. Cell Biol. 59, 267–273 (1973)
Wagner, R.C.: The effect of tannic acid on electron images of capillary endothelial cell membranes. J. Ultrastruct. Res. 57, 132–139 (1976)
Author information
Authors and Affiliations
Additional information
The Grassland Research Institute is financed through the Agricultural Research Council
Rights and permissions
About this article
Cite this article
Juniper, B.E., Lawton, J.R. The effect of caffeine, different fixation regimes and low temperature on microtubules in the cells of higher plants. Planta 145, 411–416 (1979). https://doi.org/10.1007/BF00380094
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00380094