Abstract
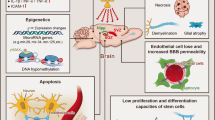
Radiation therapy is a powerful tool in the treatment of primary and metastatic cancers of the brain. However, brain tissue tolerance is limited, and radiation doses must be tailored to minimize deleterious effects on the nervous system. Due to improved treatments, including radiotherapy techniques, many patients with brain tumors survive longer, but they experience late effects of radiotherapy, especially cognitive decline, for which no efficient treatment is currently available. Improving the prevention and treatment of radiation-induced neurological defects first needs to better characterize radiation injuries in brain cells and tissues. Rodent models have been widely used for this.
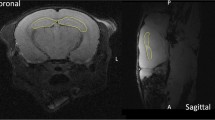
Here, observations from patients will be reviewed briefly as an introduction, mainly regarding clinical cognitive defects and anatomical alterations using magnetic resonance imaging (MRI). This limited descriptive clinical knowledge addresses many questions that arise in preclinical models regarding understanding the mechanism of radiation-induced brain dysfunction. From this perspective, we next present methods to characterize radiation-induced neurogenesis alterations in adult mice and then detail how MRI could be used as a powerful tool to explore these alterations.
Laura Mouton and Monica Ribeiro are co-first authors.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Durand T, Bernier M-O, Léger I et al (2015) Cognitive outcome after radiotherapy in brain tumor. Curr Opin Oncol 27:510. https://doi.org/10.1097/CCO.0000000000000227
Soussain C, Ricard D, Fike JR et al (2009) CNS complications of radiotherapy and chemotherapy. Lancet 374:1639–1651
Mahajan A, Dong L, Prabhu S et al (2007) Application of deformable image registration to hippocampal doses and neurocognitive outcomes. Neuro-Oncology 9:538
Seibert TM, Karunamuni R, Bartsch H et al (2017) Radiation dose–dependent hippocampal atrophy detected with longitudinal volumetric magnetic resonance imaging. Int J Radiat Oncol 97:263–269. https://doi.org/10.1016/j.ijrobp.2016.10.035
Seibert TM, Karunamuni R, Kaifi S et al (2017) Cerebral cortex regions selectively vulnerable to radiation dose-dependent atrophy. Int J Radiat Oncol 97:910–918. https://doi.org/10.1016/j.ijrobp.2017.01.005
Omuro AMP, Ben-Porat LS, Panageas KS et al (2005) Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol:62. https://doi.org/10.1001/archneur.62.10.1595
Connor M, Karunamuni R, McDonald C et al (2016) Dose-dependent white matter damage after brain radiotherapy. Radiother Oncol 121:209–216. https://doi.org/10.1016/j.radonc.2016.10.003
Connor M, Karunamuni R, McDonald C et al (2017) Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother Oncol 123:209–217. https://doi.org/10.1016/j.radonc.2017.04.006
Doetsch F (2003) A niche for adult neural stem cells. Curr Opin Genet Dev 13:543–550. https://doi.org/10.1016/j.gde.2003.08.012
Capdevila C, Vázquez LR, Martí J (2017) Glioblastoma multiforme and adult neurogenesis in the ventricular-subventricular zone: a review. J Cell Physiol 232:1596–1601. https://doi.org/10.1002/jcp.25502
Gil-Perotin S, Marin-Husstege M, Li J et al (2006) Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci 26:1107–1116. https://doi.org/10.1523/JNEUROSCI.3970-05.2006
Gupta T, Nair V, Paul SN et al (2012) Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neuro-Oncol 109:195–203. https://doi.org/10.1007/s11060-012-0887-3
Khalifa J, Tensaouti F, Lusque A et al (2017) Subventricular zones: new key targets for glioblastoma treatment. Radiat Oncol 12. https://doi.org/10.1186/s13014-017-0791-2
Bompaire F, Lahutte M, Buffat S et al (2018) New insights in radiation-induced leukoencephalopathy: a prospective cross-sectional study. Support Care Cancer 26:4217–4226. https://doi.org/10.1007/s00520-018-4296-9
Doolittle ND, Korfel A, Lubow MA et al (2013) Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 81:84–92. https://doi.org/10.1212/WNL.0b013e318297eeba
Ricard D, Idbaih A, Ducray F et al (2012) Primary brain tumours in adults. Lancet 379:1984–1996. https://doi.org/10.1016/S0140-6736(11)61346-9
Vigliani MC, Duyckaerts C, Hauw JJ et al (1999) Dementia following treatment of brain tumors with radiotherapy administered alone or in combination with nitrosourea-based chemotherapy: a clinical and pathological study. J Neuro-Oncol 41:137–149
Dropcho EJ (2010) Neurotoxicity of radiation therapy. Neurol Clin 28:217–234. https://doi.org/10.1016/j.ncl.2009.09.008
Ricard D, Soussain C, Psimaras D (2011) Neurotoxicity of the CNS: diagnosis, treatment and prevention. Rev Neurol (Paris) 167:737–745
Tofilon PJ, Fike JR (2000) The radioresponse of the central nervous system: a dynamic process. Radiat Res 153:357–370. https://doi.org/10.1667/0033-7587(2000)153[0357:TROTCN]2.0.CO;2
Fike JR (2011) Physiopathology of radiation-induced neurotoxicity. Rev Neurol (Paris) 167:746–750. https://doi.org/10.1016/j.neurol.2011.07.005
Lai R, Abrey LE, Rosenblum MK, DeAngelis LM (2004) Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology 62:451–456
El Waly B, Macchi M, Cayre M, Durbec P (2014) Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci 8:145. https://doi.org/10.3389/fnins.2014.00145
Hebb AO, Cusimano MD (2001) Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 49:1166–1184.; discussion 1184-1186. https://doi.org/10.1097/00006123-200111000-00028
Yoneoka Y, Satoh M, Akiyama K et al (1999) An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol 72:1196–1201. https://doi.org/10.1259/bjr.72.864.10703477
Monje ML, Mizumatsu S, Fike JR, Palmer TD (2002) Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955. https://doi.org/10.1038/nm749
Monje ML, Vogel H, Masek M et al (2007) Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol 62:515–520. https://doi.org/10.1002/ana.21214
Obernier K, Alvarez-Buylla A (2019) Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 146:dev156059. https://doi.org/10.1242/dev.156059
Doetsch F, Caillé I, Lim DA et al (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703–716. https://doi.org/10.1016/S0092-8674(00)80783-7
Kempermann G, Gage FH, Aigner L et al (2018) Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23:25–30. https://doi.org/10.1016/j.stem.2018.04.004
Sanai N, Nguyen T, Ihrie RA et al (2011) Corridors of migrating neurons in human brain and their decline during infancy. Nature 478:382–386. https://doi.org/10.1038/nature10487
Sanai N, Tramontin AD, Quiñones-Hinojosa A et al (2004) Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740. https://doi.org/10.1038/nature02301
Fuentealba LC, Rompani SB, Parraguez JI et al (2015) Embryonic origin of postnatal neural stem cells. Cell 161:1644–1655. https://doi.org/10.1016/j.cell.2015.05.041
Furutachi S, Miya H, Watanabe T et al (2015) Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci 18:657–665. https://doi.org/10.1038/nn.3989
Kippin TE, Martens DJ, van der Kooy D (2005) p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev 19:756–767. https://doi.org/10.1101/gad.1272305
Molofsky AV, Pardal R, Iwashita T et al (2003) Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425:962–967. https://doi.org/10.1038/nature02060
Ottone C, Krusche B, Whitby A et al (2014) Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol 16:1045–1056. https://doi.org/10.1038/ncb3045
Mira H, Andreu Z, Suh H et al (2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7:78–89. https://doi.org/10.1016/j.stem.2010.04.016
Zawadzka M, Rivers LE, Fancy SPJ et al (2010) CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6:578–590. https://doi.org/10.1016/j.stem.2010.04.002
Picard-Riera N, Decker L, Delarasse C et al (2002) Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A 99:13211–13216. https://doi.org/10.1073/pnas.192314199
Tepavčević V, Lazarini F, Alfaro-Cervello C et al (2011) Inflammation-induced subventricular zone dysfunction leads to olfactory deficits in a targeted mouse model of multiple sclerosis. J Clin Invest 121:4722–4734. https://doi.org/10.1172/JCI59145
Parras CM, Galli R, Britz O et al (2004) Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J 23:4495–4505. https://doi.org/10.1038/sj.emboj.7600447
Codega P, Silva-Vargas V, Paul A et al (2014) Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 82:545–559. https://doi.org/10.1016/j.neuron.2014.02.039
Beckervordersandforth R, Tripathi P, Ninkovic J et al (2010) In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell 7:744–758. https://doi.org/10.1016/j.stem.2010.11.017
Mich JK, Signer RA, Nakada D et al (2014) Prospective identification of functionally distinct stem cells and neurosphere-initiating cells in adult mouse forebrain. elife 3. https://doi.org/10.7554/eLife.02669
Daynac M, Chicheportiche A, Pineda JR et al (2013) Quiescent neural stem cells exit dormancy upon alteration of GABAAR signaling following radiation damage. Stem Cell Res 11:516–528. https://doi.org/10.1016/j.scr.2013.02.008
Daynac M, Morizur L, Kortulewski T et al (2015) Cell sorting of neural stem and progenitor cells from the adult mouse subventricular zone and live-imaging of their cell cycle dynamics. J Vis Exp. https://doi.org/10.3791/53247
Morizur L, Chicheportiche A, Gauthier LR et al (2018) Distinct molecular signatures of quiescent and activated adult neural stem cells reveal specific interactions with their microenvironment. Stem Cell Rep 11:565–577. https://doi.org/10.1016/j.stemcr.2018.06.005
Daynac M, Morizur L, Chicheportiche A et al (2016) Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci Rep:6. https://doi.org/10.1038/srep21505
Daynac M, Pineda JR, Chicheportiche A et al (2014) TGFβ lengthens the G1 phase of stem cells in aged mouse brain. Stem Cells 32:3257–3265. https://doi.org/10.1002/stem.1815
Alam MJ, Kitamura T, Saitoh Y et al (2018) Adult neurogenesis conserves hippocampal memory capacity. J Neurosci 38:6854–6863. https://doi.org/10.1523/JNEUROSCI.2976-17.2018
Santarelli L, Saxe M, Gross C et al (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. https://doi.org/10.1126/science.1083328
Lazarini F, Mouthon M-A, Gheusi G et al (2009) Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One 4. https://doi.org/10.1371/journal.pone.0007017
Feierstein CE, Lazarini F, Wagner S et al (2010) Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front Behav Neurosci 4. https://doi.org/10.3389/fnbeh.2010.00176
Valley MT, Mullen TR, Schultz LC et al (2009) Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci 3. https://doi.org/10.3389/neuro.22.003.2009
Amano T, Inamura T, Wu C-M et al (2002) Effects of single low dose irradiation on subventricular zone cells in juvenile rat brain. Neurol Res 24:809–816. https://doi.org/10.1179/016164102101200771
Hellström NAK, Björk-Eriksson T, Blomgren K, Kuhn HG (2009) Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells 27:634–641. https://doi.org/10.1634/stemcells.2008-0732
Fukuda H, Fukuda A, Zhu C et al (2004) Irradiation-induced progenitor cell death in the developing brain is resistant to erythropoietin treatment and caspase inhibition. Cell Death Differ 11:1166. https://doi.org/10.1038/sj.cdd.4401472
Osato K, Sato Y, Ochiishi T et al (2010) Apoptosis-inducing factor deficiency decreases the proliferation rate and protects the subventricular zone against ionizing radiation. Cell Death Dis 1:e84. https://doi.org/10.1038/cddis.2010.63
Baser A, Skabkin M, Kleber S et al (2019) Onset of differentiation is post-transcriptionally controlled in adult neural stem cells. Nature 566:100–104. https://doi.org/10.1038/s41586-019-0888-x
Achanta P, Capilla-Gonzalez V, Purger D et al (2012) Subventricular zone localized irradiation affects the generation of proliferating neural precursor cells and the migration of neuroblasts. Stem Cells 30:2548–2560. https://doi.org/10.1002/stem.1214
Capilla-Gonzalez V, Guerrero-Cazares H, Bonsu JM et al (2014) The subventricular zone is able to respond to a demyelinating lesion after localized radiation. Stem Cells 32:59–69. https://doi.org/10.1002/stem.1519
Panagiotakos G, Alshamy G, Chan B et al (2007) Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One 2:e588. https://doi.org/10.1371/journal.pone.0000588
Begolly S, Olschowka JA, Love T et al (2018) Fractionation enhances acute oligodendrocyte progenitor cell radiation sensitivity and leads to long term depletion. Glia 66:846–861. https://doi.org/10.1002/glia.23288
Pineda JR, Daynac M, Chicheportiche A et al (2013) Vascular-derived TGF-β increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol Med 5:548–562. https://doi.org/10.1002/emmm.201202197
Monje ML, Palmer T (2003) Radiation injury and neurogenesis. Curr Opin Neurol 16:129–134. https://doi.org/10.1097/01.wco.0000063772.81810.b7
de Graaf RA (2019) In vivo NMR spectroscopy: principles and techniques, 3rd edn. John Wiley & Sons, New York
Le Bihan D (1995) Magnetic resonance imaging of diffusion and perfusion: applications to functional imaging. Raven Press, New York
Haacke EM (1999) Magnetic resonance imaging; physical principles and sequence design. Wiley, New York
Jahng G-H, Li K-L, Ostergaard L, Calamante F (2014) Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol 15:554. https://doi.org/10.3348/kjr.2014.15.5.554
Petcharunpaisan S, Ramalho J, Castillo M (2010) Arterial spin labeling in neuroimaging. World J Radiol 2:384–398. https://doi.org/10.4329/wjr.v2.i10.384
Le Bihan D (2019) What can we see with IVIM MRI? NeuroImage 187:56–67. https://doi.org/10.1016/j.neuroimage.2017.12.062
Le Bihan D, Breton E (1985) Imagerie de diffusion in-vivo par résonance magnétique nucléaire. Comptes-Rendus de l'Académie des Sciences 93:27–34
Le Bihan D (2014) Diffusion MRI: what water tells us about the brain. EMBO Mol Med 6:569–573
Iima M, Le Bihan D (2015) Clinical Intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 278:13–32. https://doi.org/10.1148/radiol.2015150244
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23:698–710. https://doi.org/10.1002/nbm.1518
Le Bihan D, Mangin J-F, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546. https://doi.org/10.1002/jmri.1076
Ogawa S, Lee TM, Nayak AS, Glynn P (1990) Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 14:68–78
Keilholz SD, Pan W-J, Billings J et al (2017) Noise and non-neuronal contributions to the BOLD signal: applications to and insights from animal studies. NeuroImage 154:267–281. https://doi.org/10.1016/j.neuroimage.2016.12.019
Prost RW (2008) Magnetic resonance spectroscopy. Med Phys 35:4530–4544. https://doi.org/10.1118/1.2975225
Jiang X, Yuan L, Engelbach JA et al (2015) A gamma-knife-enabled mouse model of cerebral single-hemisphere delayed radiation necrosis. PLoS One 10:e0139596. https://doi.org/10.1371/journal.pone.0139596
Wadghiri YZ, Blind JA, Duan X et al (2004) Manganese-enhanced magnetic resonance imaging (MEMRI) of mouse brain development. NMR Biomed 17:613–619. https://doi.org/10.1002/nbm.932
Gazdzinski LM, Cormier K, Lu FG et al (2012) Radiation-induced alterations in mouse brain development characterized by magnetic resonance imaging. Int J Radiat Oncol Biol Phys 84:e631–e638. https://doi.org/10.1016/j.ijrobp.2012.06.053
Nieman BJ, de Guzman AE, Gazdzinski LM et al (2015) White and gray matter abnormalities after cranial radiation in children and mice. Int J Radiat Oncol Biol Phys 93:882–891. https://doi.org/10.1016/j.ijrobp.2015.07.2293
Trivedi R, Khan AR, Rana P et al (2012) Radiation-induced early changes in the brain and behavior: serial diffusion tensor imaging and behavioral evaluation after graded doses of radiation. J Neurosci Res 90:2009–2019. https://doi.org/10.1002/jnr.23073
Verreet T, Quintens R, Van Dam D et al (2015) A multidisciplinary approach unravels early and persistent effects of X-ray exposure at the onset of prenatal neurogenesis. J Neurodev Disord 7. https://doi.org/10.1186/1866-1955-7-3
Verreet T, Rangarajan JR, Quintens R et al (2016) Persistent impact of in utero irradiation on mouse brain structure and function characterized by MR imaging and Behavioral analysis. Front Behav Neurosci 10:83. https://doi.org/10.3389/fnbeh.2016.00083
Pérès EA, Etienne O, Grigis A et al (2018) Longitudinal study of irradiation-induced brain microstructural alterations with S-index, a diffusion MRI biomarker, and MR spectroscopy. Int J Radiat Oncol Biol Phys 102:1244–1254. https://doi.org/10.1016/j.ijrobp.2018.01.070
de Guzman AE, Gazdzinski LM, Alsop RJ et al (2015) Treatment age, dose and sex determine neuroanatomical outcome in irradiated juvenile mice. Radiat Res 183:541–549. https://doi.org/10.1667/RR13854.1
Kumar M, Haridas S, Trivedi R et al (2013) Early cognitive changes due to whole body γ-irradiation: a behavioral and diffusion tensor imaging study in mice. Exp Neurol 248:360–368. https://doi.org/10.1016/j.expneurol.2013.06.005
Gupta M, Mishra SK, Kumar BSH et al (2017) Early detection of whole body radiation induced microstructural and neuroinflammatory changes in hippocampus: a diffusion tensor imaging and gene expression study. J Neurosci Res 95:1067–1078. https://doi.org/10.1002/jnr.23833
Constanzo J, Dumont M, Lebel R et al (2018) Diffusion MRI monitoring of specific structures in the irradiated rat brain. Magn Reson Med 80:1614–1625. https://doi.org/10.1002/mrm.27112
Serduc R, van de Looij Y, Francony G et al (2008) Characterization and quantification of cerebral edema induced by synchrotron x-ray microbeam radiation therapy. Phys Med Biol 53:1153–1166. https://doi.org/10.1088/0031-9155/53/5/001
Watve A, Gupta M, Khushu S, Rana P (2018) Longitudinal changes in gray matter regions after cranial radiation and comparative analysis with whole body radiation: a DTI study. Int J Radiat Biol 94:532–541. https://doi.org/10.1080/09553002.2018.1466064
Saito S, Sawada K, Mori Y et al (2015) Brain and arterial abnormalities following prenatal X-ray irradiation in mice assessed by magnetic resonance imaging and angiography. Congenit Anom 55:103–106. https://doi.org/10.1111/cga.12101
Constanzo J, Masson-Côté L, Tremblay L et al (2017) Understanding the continuum of radionecrosis and vascular disorders in the brain following gamma knife irradiation: an MRI study. Magn Reson Med 78:1420–1431. https://doi.org/10.1002/mrm.26546
Herynek V, Burian M, Jirák D et al (2004) Metabolite and diffusion changes in the rat brain after Leksell gamma knife irradiation. Magn Reson Med 52:397–402. https://doi.org/10.1002/mrm.20150
Gupta M, Rana P, Trivedi R et al (2013) Comparative evaluation of brain neurometabolites and DTI indices following whole body and cranial irradiation: a magnetic resonance imaging and spectroscopy study. NMR Biomed 26:1733–1741. https://doi.org/10.1002/nbm.3010
Kovács N, Szigeti K, Hegedűs N et al (2018) Multimodal PET/MRI imaging results enable monitoring the side effects of radiation therapy. Contrast Media Mol Imaging, In. https://www.hindawi.com/journals/cmmi/2018/5906471/. Accessed 29 May 2019
Chan KC, Khong P-L, Cheung MM et al (2009) MRI of late microstructural and metabolic alterations in radiation-induced brain injuries. J Magn Reson Imaging 29:1013–1020. https://doi.org/10.1002/jmri.21736
Bálentová S, Hnilicová P, Kalenská D et al (2017) Effect of whole-brain irradiation on the specific brain regions in a rat model: metabolic and histopathological changes. Neurotoxicology 60:70–81. https://doi.org/10.1016/j.neuro.2017.03.005
Yamaguchi M, Saito H, Suzuki M, Mori K (2000) Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. NeuroReport 11 (9):1991-1996
Couillard-Despres S, Finkl R, Winner B et al (2008) In vivo optical imaging of neurogenesis: watching new neurons in the intact brain. Mol Imaging 7. https://doi.org/10.2310/7290.2008.0004
Leung HWC, Chan ALF, Chang MB (2016) Brain dose-sparing radiotherapy techniques for localized intracranial germinoma: Case report and literature review of modern irradiation. Cancer Radiother 20:210-216. https://doi.org/10.1016/j.canrad.2016.02.007
Kazda T, Jancalek R, Pospisil P et al (2014) Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol 9:139. https://doi.org/10.1186/1748-717X-9-139
Welzel G, Fleckenstein K, Schaefer J et al (2008) Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 72:1311–1318. https://doi.org/10.1016/j.ijrobp.2008.03.009
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Mouton, L. et al. (2021). Experimental and Preclinical Tools to Explore the Main Neurological Impacts of Brain Irradiation: Current Insights and Perspectives. In: Seano, G. (eds) Brain Tumors. Neuromethods, vol 158. Springer, New York, NY. https://doi.org/10.1007/978-1-0716-0856-2_11
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0856-2_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-0716-0855-5
Online ISBN: 978-1-0716-0856-2
eBook Packages: Springer Protocols