Abstract
Ammonia is a crucial hydrogen carrier. This paper explores small-scale green ammonia production from two-stage sorption-enhanced gasification of biomass. ASPEN Plus was used to calculate the mass and energy balance of two novel process designs; configuration (a) employs fractional flue gas recycling to supply nitrogen for producing carbon-neutral ammonia without air separation, and configuration (b) uses air separation to produce carbon-negative ammonia by efficiently capturing CO2. The mass balance shows that the ammonia yield of configuration (a) is about 5% higher than that of configuration (b). The energy analysis shows that additional biomass fed to the combustor is the primary energy penalty in configuration (a), while the primary energy penalty in configuration (b) is from the electricity consumption by the ASU and CO2 compressor. The energy penalty for configuration (a) is considerably higher than that for configuration (b); hence, configuration (b) has lower energy consumption.(36.4 GJ/t NH3 vs. 40.2 GJ/t NH3). Overall, configuration (b) is superior to configuration (a) from a techno-environmental standpoint.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
About 27% of the hydrogen produced annually is used for producing ammonia via the Haber-Bosch process [1]. Ammonia is a vital hydrogen carrier due to its high hydrogen content(18 wt%) and relatively low energy demand for liquefaction. The maturity and flexibility of ammonia supply chains are set to make ammonia a key player in low-carbon economies [2]. Currently, blue or grey hydrogen from coal and natural gas and nitrogen air separation is used for ammonia synthesis. Hydrogen production accounts for most of the energy consumed in ammonia plants. Existing ammonia production routes have the following shortcomings: (i) high carbon footprint, accounting for up to 1.6% of annual global CO2 emissions [3], (ii) rigorous and energy-intensive syngas cleanup to meet the standards required for the synthesis loop.
Green ammonia from biomass gasification has recently received interest as a pathway for lowering the carbon footprint. Carbon-negative ammonia can be produced from biomass when carbon capture is employed. This study evaluates simplified and efficient biomass conversion to ammonia using two-stage sorption-enhanced gasification (SEG). Two processes for small-scale, decentralized ammonia production are analyzed using ASPEN Plus simulation. One process produces carbon-neutral ammonia, while the other produces negative-carbon ammonia by capturing CO2.
2 Process Design and Simulation
2.1 Process Design
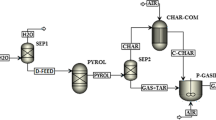
The proposed process is based on two-stage SEG of biomass. As shown in Fig. 1, the first stage involves steam gasification of biomass in a fluidized bed gasifier at 650–750 ℃. Then the product gas from the gasifier undergoes sorption-enhanced reforming at 550–650 ℃ in an autothermal moving bed reformer with gas-solid countercurrent contacting. Continuous in situ separation of CO2 by CaO (carbonation) in the gasifier and reformer promotes the WGS, resulting in a hydrogen-rich product gas with > 90%mol hydrogen(dry basis) [4,5,6]. CaO also catalyzes tar reforming. The CaCO3 from the gasifier and reformer is decomposed back to CaO in a fluidized bed combustor using heat from char and supplementary fuel combustion.
Figure 1 shows two routes for ammonia production. In configuration (a), part of the flue gas (mainly N2 and CO2) is introduced into the moving bed reformer, where CaO recaptures CO2, and the N2 required for ammonia synthesis is released into the syngas. The size of the flue gas recycle is adjusted such that H2/N2 is close to 3. Employing a flue gas recycle to supply nitrogen takes advantage of in-situ carbon capture by CaO to separate N2 and CO2, effectively eliminating the need for an air separation unit (ASU). After compression, methanation and temperature-swing adsorption (TSA) convert and remove oxygen compounds that can poison ammonia synthesis catalysts. Configuration (b) uses an ASU to supply nitrogen. Oxygen is used for combustion to capture CO2. After compression, pressure-swing adsorption (PSA) removes almost all traces to produce pure hydrogen. The PSA tail gas supplement fuel for the combustor. The low inert content of syngas lessens the purge size for configuration (b) compared to configuration (a).
Ammonia synthesis uses a low-pressure loop (82 atm) based on the ICI leading concept for small-scale, modular ammonia production [7]. The purge gas from the synthesis loop supplements fuel for the combustor in both configurations. Waste heat recovered throughout the plant generates electricity in a three-stage steam turbine [8]. Steam for gasification and reforming is drawn after the medium-pressure turbine.
2.2 ASPEN Plus Simulation
This study considered a decentralized small-scale plant processing at least 128 tonnes per day (t/d) of dried wheat stalks (Table 1). This translates to 24 MWth of fuel fed to the gasifier. The mass and energy balance of the two designs was calculated using ASPEN Plus under steady-state conditions. Physical properties and thermodynamic relationships were calculated using steam tables and the Peng–Robinson equation of state with the Boston-Mathias modifications (PR-BM).
The operating parameters of each unit operation are shown in Fig. 1. The simulation used four-stage compressors with inter-stage cooling. The isentropic and mechanical efficiency of the compressors, pumps and turbines were assumed to be 80% and 95%, respectively. A 10 ℃ minimum temperature approach was assumed for all heat exchangers. The hydrogen recovery rate during PSA was assumed to be 85% [9]. Water removal by TSA is assumed to be 99.99% [10].
3 Results and Discussion
3.1 Mass Balance
The mass balance of the two configurations is summarized in Table 2. The flow rates and product distribution for the gasifier are identical for both processes. 53.1 t/d (40,545 Nm3/h) of syngas are produced from the gasifier. The product gas composition is comparable to pilot-scale results for SEG [12]. The steam fed to the reformer is also equal due to identical raw syngas compositions. Hence, almost equivalent hydrogen yields are obtained from both cases. The calcium oxide flow rate of configuration (a) is double that of configuration (b) due to the additional CO2 introduced by recycling 28% of the flue gas to the reformer. This higher sorbent load for configuration (a) results in a supplementary fuel demand for the combustor that is nearly six times higher than in configuration (b). Configuration (a) emits 3.1 tonnes of CO2 per tonne of NH3, while configuration (b) captures > 99% of the total carbon fed into the process.
After syngas cleanup, the syngas contains almost no oxygen compounds. Around 380 t/d and 353 t/d of syngas are fed to the synthesis loop of configuration (a) and (b), respectively. The hydrogen conversion in the synthesis loop is slightly higher for configuration (b) because of a lower inert content (6.4% vs. 1%). The overall ammonia yield from configuration (a) is 5% higher than from configuration (b) due to the higher syngas feed in the former.
3.2 Energy Balance
Table 3 shows the overall energy balance for the two process designs. As seen in the material balance (Table 2), the supplementary fuel demand for configuration (a) is higher; thus, the total biomass input of configuration (a) is about 16% higher than for configuration (b). Meanwhile, the electricity demand for configuration (b) is 40% higher than for configuration (b). The relatively higher power demand is mainly due to electricity used in the ASU and CO2 compressor. The overall energy input of configuration (a) is higher than configuration (b).
The energy output in ammonia is higher for configuration (a) due to a higher overall yield (see Table 2). As shown in Fig. 1, configuration (a) has an extra stream available for waste heat recovery. Hence, configuration (a) generates more electricity than configuration (b). It should be noted both processes have a power deficit; 300 kWe for configuration (a) and 2341 kWe for configuration (b).
The biomass-to-ammonia efficiency of configuration (a) is relatively lower due to its high biomass requirement. Likewise, the biomass-to-ammonia + power efficiency of configuration (b) is relatively lower due to its high power demand. Therefore, the primary energy penalty in configuration (a) is from the combustor, while the primary energy penalty in configuration (b) is from the air separation and CO2 compression.
The efficiency of ammonia production is typically measured by the net energy consumption per tonne of ammonia. As shown in Table 3, configuration (a) consumes 40.2 GJ to produce one tonne of ammonia. Configuration (b) consumes 36.4 GJ per tonne of ammonia. This indicates that the energy penalty of configuration (a) is higher than configuration (b). Therefore, better energy efficiency clearly makes configuration (b) the superior option.
It is worth mentioning that the energy consumption of the two configurations is significantly lower than processes based on coal gasification (42 GJ/t NH3), electrolysis (43 GJ/t NH3), biogas reforming (42 GJ/t NH3) and biomass oxy-gasification (58 GJ/t NH3) [13]. However, the proposed processes remain inferior to modern methane reforming plants (28 GJ/t NH3).
4 Conclusions
This study used ASPEN Plus to analyze the mass and energy balance of two novel designs for small-scale green ammonia production using two-stage SEG of biomass. Configuration (a) uses flue gas recycling to produce carbon-neutral ammonia without air separation. Configuration (b) uses air separation to produce carbon-negative by capturing CO2.
Results showed that the syngas and ammonia yield of configuration (a) is higher than configuration (b). Configuration (a) emits 3.1 tonnes of CO2 per tonne of NH3, while configuration (b) captures > 99% of the total carbon fed into the process. The primary energy penalty in configuration (a) is from additional fuel for sorbent regeneration, while the primary energy penalty in configuration (b) is from the ASU and CO2 compressor. The energy penalty for configuration (b) is lower than for configuration (a); thus, configuration (b) retains a higher energy efficiency. Therefore, in addition to a lower carbon footprint, better energy efficiency makes configuration (b) the superior process.
References
IEA: The Future of Hydrogen-Seizing Today’s Opportunities (2019)
Thomas, G., Parks, G.: Potential Roles of Ammonia in a Hydrogen Economy (2006)
Rouwenhorst, K.H.R., Krzywda, P.M., Benes, N.E., et al.: Ammonia Production Technologies. Techno-Economic Challenges of Green Ammonia as an Energy Vector, pp. 41–83 (2021)
Chen, S., Wang, D., Xue, Z., et al.: Calcium looping gasification for high-concentration hydrogen production with CO2 capture in a novel compact fluidized bed: Simulation and operation requirements. Int. J. Hydrogen Energy 36(8), 4887–4899 (2011)
Zhao, H., Song, G., Shen, L., et al.: Novel technique route of coal gasification with CO2 capture using CaO sorbents via three-stage interconnected fluidized beds. Energy Fuels 26(5), 2934–2941 (2012)
Li, B., Fabrice Magoua Mbeugang, C., Liu, D., et al.: Simulation of sorption enhanced staged gasification of biomass for hydrogen production in the presence of calcium oxide. Int. J. Hydrogen Energy 45(51), 26855–26864 (2020)
Arora, P., Sharma, I., Hoadley, A., et al.: Remote, small-scale, ‘greener’ routes of ammonia production. J. Clean. Prod. 199, 177–192 (2018)
Spath, P., Aden, A., Eggeman, T., et al.: Biomass to Hydrogen Production Detailed Design and Economics Utilizing the Battelle Columbus Laboratory Indirectly-Heated Gasifier (2005)
Luberti, M., Ahn, H.: Review of Polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 47(20), 10911–10933 (2022)
Kemper, J., Sutherland, L., Watt, J., et al.: Evaluation and analysis of the performance of dehydration units for CO2 capture. Energy Procedia 63, 7568–7584 (2014)
Lu, Y., Guo, L., Ji, C., et al.: Hydrogen production by biomass gasification in supercritical water: a parametric study. Int. J. Hydrogen Energy 31(7), 822–831 (2006)
Fuchs, J., Schmid, J.C., Müller, S., et al.: Dual fluidized bed gasification of biomass with selective carbon dioxide removal and limestone as bed material: a review. Renew. Sustain. Energy Rev. 107, 212–231 (2019)
Tunå, P., Hulteberg, C., Ahlgren, S.: Techno-economic assessment of nonfossil ammonia production. Environ. Prog. Sustain. Energy 33(4), 1290–1297 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this paper
Cite this paper
Dziva, G., Zeng, L. (2024). Process Simulation of Green Ammonia Production Via Sorption-Enhanced Gasification of Biomass. In: Sun, H., Pei, W., Dong, Y., Yu, H., You, S. (eds) Proceedings of the 10th Hydrogen Technology Convention, Volume 1. WHTC 2023. Springer Proceedings in Physics, vol 393. Springer, Singapore. https://doi.org/10.1007/978-981-99-8631-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-99-8631-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8630-9
Online ISBN: 978-981-99-8631-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)