Abstract
The iodine-sulfur cycle coupled with a high-temperature gas-cooled reactor is a clean and efficient hydrogen production technology. The sulfuric acid decomposition reaction is the highest temperature process in the iodine-sulfur cycle, which requires 850 °C high temperature and catalyst to carry out at a high conversion rate. This study prepared a series of loaded sulfuric acid decomposition catalysts using anatase TiO2 and Ta2O5 as catalyst carriers and precious metal Pt as the active component. XRD, BET, and ICP-MS characterization of the catalysts demonstrated that the high calcination temperature could increase the crystallinity and content of the active components and decrease the specific surface area of the catalysts. The Pt/TiO2-850 catalyst showed good performance under different feed concentrations, reaction temperatures, and particle sizes. In addition, the scale-up production does not affect the Pt/TiO2-850 catalyst reaction performance. The Pt/TiO2-850 catalyst was tested in a bayonet-tube SiC reactor with a 100-h high throughput lifetime, which proved that the Pt/TiO2-850 catalyst has good stability.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Hydrogen is an important industrial raw material and an ideal energy carrier in the future, it is easy to store and transport as a secondary energy source and can be used directly as a fuel [1]. In addition to the traditional ammonia synthesis, methanol synthesis, and petroleum refining, hydrogen can be utilized on a large scale in hydrogen metallurgy, coal liquefaction, and fuel cell vehicles, providing the necessary technological support for these industries to reduce carbon emissions [2,3,4]. The utilization of nuclear energy for hydrogen production can achieve efficient, large-scale, and carbon-emission-free.
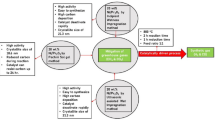
The hydrogen production technologies from nuclear energy using water as feedstock mainly include thermochemical cycles and high-temperature steam electrolysis. However, the iodine-sulfur (IS) cycle coupled with high-temperature gas-cooled reactors (HTGR) is considered the most suitable and promising hydrogen production technologies, which have been widely studied [5,6,7,8]. The IS cycle consists of three reactions, (1) the decomposition of H2SO4 into SO2, O2, and H2O; (2) the decomposition of HI into H2 and I2; and (3) the regeneration of HI and H2SO4 by the Bunsen reaction. The reaction of (1) recovered SO2 and (2) recovered I2 with H2O to form HI and H2SO4 [9]. The IS cycle process showed in Fig. 1. The sulfuric acid (SA) decomposition reaction is a key process for coupling the IS cycle with a high-temperature heat source. The reaction needs to be carried out between 800 and 1000 ℃. The SA decomposition process consists of a two-step reaction; first, SA decomposes to SO3 and H2O at about 400 ℃; second, the SO3 decomposes to SO2 and O2 above 800 ℃. The first step reaction is fast and does not require a catalyst; the second step reaction is thermodynamically favorable, but the reaction rate is slow and requires a catalyst [10].
In addition to the IS cycle, other sulfur-containing cycles such as the mixed sulfur cycle and the bromine-sulfur cycle also contain SA decomposition reactions [11,12,13]. The SA decomposition process is a reaction that utilizes high-temperature heat in these cycles, and its energy consumption has a large impact on the overall process of hydrogen production efficiency. And the catalyst performance of the SO3 decomposition process strongly affects the conversion rate and energy consumption. For this reason, a lot of research has been conducted on SO3 decomposition catalysts, which are mainly divided into precious metal catalysts and oxide catalysts. Noble metals represented by Pt show excellent catalytic performance in SA decomposition reaction, and researchers have studied the SO3 decomposition conversion rates of Pt, Pd, Rh, Ru, and Ir, and their activities are in the order of Pt > Pd > Rh > Ir > Ru [14]. Noble metal catalysts have good catalytic activity for SO3 decomposition, but they are more expensive. For the consideration of economics and cost of hydrogen production under large-scale hydrogen production conditions, researchers have started to investigate non-precious metal catalysts. Non-precious metal catalysts mainly include oxides of Cr, Fe, Cu, Ce, V, Mn, Co, Zn, Al, etc., and studies have shown that the order of activity of various metal oxides is Fe2O3 > V2O5 > CuO > MnO2 > Cr2O3 > CeO2 > CoO > ZnO > Al2O3 [15]. These metal oxides are inexpensive but have an overall low catalytic activity. In order to further improve the conversion efficiency of oxide catalysts, researchers began to study composite metal oxides, and have explored the reaction activity of Fe/Al, Fe/Ti, Fe/Cu, Fe/Cr, Cr/Cu, Cu/V, Ni/Fe, and other composite metal oxides [16,17,18,19,20]. The results show that these composite metal oxides can present high catalytic activity, some of them are even higher than the noble metal Pt, but their stability is generally poor. The reason is that during the long-time reaction process, the composite metal oxides decompose to generate monometallic oxides, and some even generate metal sulfates, which volatilize and agglomerate and thus lose catalytic activity. The catalysts for industrial applications need not only high catalytic activity but also good reaction stability to ensure the stable operation of industrial processes. All things considered, the development of catalysts for industrial applications ultimately requires the choice of noble metal catalysts. In order to reduce the price of noble metal catalysts and reduce the cost of hydrogen production, researchers loaded Pt on Al2O3, BaSO4, SiC, and rutile phase TiO2 carriers to study their reaction activity and stability, and the results showed that the carrier of Pt has an important influence on the activity and stability of the catalyst [21,22,23,24,25]. Because the catalysts are in a SA vapor environment, although Pt has good stability, the substrates are prone to sulfation and poisoning. Therefore, the carrier matrix loaded with Pt must be free from sulfation at high temperatures and have very good stability.
By testing different oxide carriers, researchers found that anatase TiO2 and Ta2O5 have better stability at low temperatures (600 ℃) but lower conversion efficiency [26, 27]. To effectively utilize the HTGR process heat for efficient hydrogen production, the SA decomposition process in the IS cycle needs to be carried out at about 850 ℃, a temperature that ensures that the SA decomposition has a high decomposition efficiency and matches the exit temperature of the HTGR [28,29,30]. Second, the SA decomposition process is affected by various factors under the closed operation of the IS cycle, and the catalytic performance of the SA decomposition catalyst under different reaction conditions needs to be investigated. In addition, the long-term reaction stability of the catalyst is a prerequisite to ensure that the catalyst can be further applied, and the reaction lifetime of the catalyst needs to be tested. Based on this, a series of different SA decomposition catalysts were prepared with Pt-loaded anatase TiO2 and Ta2O5, and their compositional analysis, structural characterization, and surface properties were investigated. The catalysts were tested for their SA decomposition performance at high temperatures, and suitable SA decomposition catalysts were selected. The selected catalysts were tested under different reaction conditions to comprehensively evaluate their reaction performance. Finally, the catalysts were scaled up for production and tested for long time stability life.
2 Materials and Methods
2.1 Preparation of Catalyst
The anatase TiO2 (Ta2O5) was added to 1.0 wt% H2PtCl6 aqueous solution under stirring. After impregnation for 3 h, the suspension was dried by evaporation at 80 ℃ using a rotary evaporator. Then the dried samples were warmed up to 120 ℃ using an oven and kept for 4 h. The samples were then placed in a muffle furnace and calcined at 450 ℃ (650 ℃, 850 ℃) for 4 h. The obtained catalysts were named Pt/TiO2-450, Pt/TiO2-650, and Pt/TiO2-850 (Pt/Ta2O5-450, Pt/Ta2O5-650, and Pt/Ta2O5-850).
2.2 Analytical Instruments
X-ray diffraction (XRD) was used for phase analysis of samples, the instrument model used was Rigaku D/MAX 2500H (Japan), and the scanning range was 10–90°. The specific surface area of the catalyst was analyzed by Quanta Autosorb-iQ (USA). The metallic platinum content of the catalysts was measured using an Agilent Technologies 8800 (USA). Scanning electron microscopy (SEM) was used to analyze the catalyst surface structure, and the instrument model used was Hitachi SU-8010 (Japan).
2.3 Catalytic Activity Test
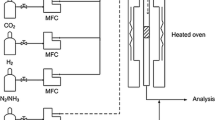
The catalytic activity of the catalyst was evaluated in a fixed bed reactor. The catalyst was loaded in the middle of the quartz reaction tube and the tubular reactor was heated using an electric heater, the temperature of which was controlled by a thermocouple and a controller. The SA was pumped into the tubular reactor using a peristaltic pump, and the bottom part of the reactor was connected to a condenser and a tail gas absorption device. At the end of the reaction, the undecomposed SA solution at the bottom outlet of the condenser was collected and its concentration was measured, and then the SA decomposition conversion of the catalyst was calculated. The SA decomposition conversion is calculated by the following equation:
where m1 and C1 denote the mass and molar mass concentration of collected SA, and m0 and C0 denote the mass and molar mass concentration of concentrated SA entering the reactor.
3 Results and Discussion
3.1 Characterization of Catalysts
There is a relationship between the structure of the catalyst and the catalytic performance. In order to investigate the conformational relationship of Pt-loaded metal oxide catalysts, XRD analysis, specific surface area, and Pt content measurements were performed on the prepared catalysts to analyze their structures and compositions. Figure 2 shows the XRD spectra of the catalysts. The catalysts had distinctive diffraction peaks of anatase TiO2 crystals and Ta2O5 crystals, and increasing the calcination temperature did not change the crystalline phase of the metal oxide carriers. The characteristic diffraction peaks of Pt crystals also appear in the catalysts. In the catalyst series of the same carrier, the increase of calcination temperature increases the intensity of the characteristic diffraction peaks of Pt crystals and promotes the crystallization of Pt nanoparticles. It is shown that the intensity of the characteristic diffraction peaks of Pt crystals on the catalysts with TiO2 as the carrier is stronger than that of the catalysts with Ta2O5 as the carrier. It could be concluded from this that the high temperature is favorable for the crystallization of Pt nanoparticles, and the catalyst calcined at 850 ℃ has the best crystallinity of Pt nanoparticles. Compared with the carrier Ta2O5, the Pt nanoparticles loaded on TiO2 have better crystallinity.
Specific surface area and active component content are important properties of catalysts. Table 1 shows the specific surface area and Pt content of different catalysts. It could be seen that for the catalysts with the same carrier, the specific surface area of the catalysts gradually decreased and the Pt content gradually increased with the increase of calcination temperature. The reason could be that increasing the calcination temperature induces the Pt nanoparticles to migrate and fuse, and the small particles become large particles, thus decreasing the specific surface area of the catalyst [31]. In addition, high calcination temperature could make the impurities in the catalyst volatilize and increase the Pt content in the catalyst. The specific surface area and Pt content of Pt/TiO2 series catalysts were higher than those of Pt/Ta2O5 series catalysts, which was possible because the specific surface area of Ta2O5 was smaller, which also resulted in less loading of Pt on Ta2O5 and the Pt content was lower. In general, the larger the specific surface area of the catalyst and the higher the content of active components, the higher its conversion efficiency [32]. Therefore, the reaction performance of Pt/TiO2 series catalysts is better than that of Pt/Ta2O5 series catalysts. In order to verify the characterization results, the catalysts need to be tested for SA decomposition experiments to compare their catalytic performance.
3.2 Catalytic Activities of Catalysts
The catalysts have different conversion efficiency under different reaction conditions, therefore, the catalytic activity of the catalysts should be evaluated under various reaction conditions, including feed rate, feed concentration, reaction temperature, and catalyst particle size. Considering that the feed rate has a great influence on the catalytic performance of the catalysts, this study first carried out experiments at different weight hourly space velocities (WHSV) to screen out the catalysts with the best catalytic performance for SA decomposition and then tested the performance of the screened catalysts under the reaction conditions of feed concentration, reaction temperature, and particle diameter to comprehensively evaluate the catalytic activity.
The catalytic activity of catalysts at different weight hourly space velocities. In order to screen the best catalytic performance of the SA decomposition catalyst, the decomposition efficiency of the catalyst was tested at different WHSV at 850 ℃. The results are shown in Fig. 3(a), where the decomposition efficiency of the catalyst gradually decreases as the SA feed rate increases. The faster the feed rate, the shorter the time for SO3 to contact the catalyst, thus causing the decomposition efficiency of the catalyst to decrease. When the WHSV is 13 h−1, the conversion efficiency of the catalyst is the highest. The conversion efficiency of the Pt/TiO2 series catalyst was higher than that of the Pt/Ta2O5 series catalyst, which was consistent with the results of the specific surface area and Pt content analysis. The Pt/TiO2-850 catalyst had the highest overall SA decomposition efficiency. The reason could be that the high calcination temperature induced the reduction of PtOx in the catalyst to the active component Pt atoms, thus improving the catalytic performance of Pt/TiO2. Although the specific surface area of the Pt/TiO2-850 catalyst was the smallest, the crystallinity and content of Pt nanoparticles in the Pt/TiO2-850 catalyst were the highest, indicating that the crystallinity and content of Pt nanoparticles had more influence on the catalytic performance than the specific surface area.
The catalytic activity of Pt/TiO2-850 at different feed concentrations. The SA decomposition experiments at different WHSV determined that the Pt/TiO2-850 catalyst has the highest conversion efficiency. During the experiment, 98%wt concentrated SA was used, and the feed concentration of the SA decomposition unit during the IS cycle was difficult to reach 98%wt. Even if the SA feed concentration could reach 98%wt, it would consume a lot of heat and increase the economic cost of the process. Therefore, it is necessary to explore the conversion efficiency of the catalyst under low concentration SA feed conditions. Based on this, different concentrations of SA were prepared in this study, and Pt/TiO2-850 catalyst was selected as the object of study to test the effect of SA feed concentration on the catalytic performance of the catalyst under the reaction conditions at a temperature of 850 ℃ and a WHSV of 13 h−1, and the results are shown in Fig. 3(b). When the SA feed concentration was between 50%wt and 75%wt, the conversion efficiency of Pt/TiO2-850 was around 90%; when the SA feed concentration was higher than 75%wt, the conversion efficiency of Pt/TiO2-850 started to decrease, and finally stabilized at around 80%. According to the experimental results, the SA decomposition using this catalyst during the IS cycle could concentrate the SA generated from the Bunsen reaction to 80% wt. This can ensure the catalyst has high conversion efficiency and reduce the heat of concentrated SA, thus reducing the heat consumption of the whole process.
The catalytic activity of Pt/TiO2-850 at different temperatures. The IS cycle eventually needs to be coupled with a HTGR to heat the SA decomposition process using high-temperature helium. Compared with electric furnace heating, high-temperature helium heating would cause heating instability problems and does not ensure that the catalyst bed temperature is always maintained at 850 ℃. Therefore, the effect of heating temperature on the catalytic performance of Pt/TiO2-850 is required to be investigated. The temperature range of 500–850 ℃ was selected to test the SA decomposition conversion of Pt/TiO2-850 catalyst, and the experimental results were compared with the theoretical equilibrium conversion of the SA decomposition reaction, and the results are shown in Fig. 3(c) [33]. As the temperature increases, the conversion efficiency of the catalyst increases gradually, which is consistent with the fact that SA decomposition is a thermally absorbing reaction. In the temperature range of 750–850 ℃, the difference between the conversion rate of the catalyst and the equilibrium conversion rate is around 10%; when the temperature is lower than 750 ℃, the difference between the conversion rate of the catalyst and the equilibrium conversion rate is around 20%, and the high temperature is favorable to the Pt/TiO2-850-catalyzed SA decomposition reaction. In order to ensure the SA decomposition with high conversion efficiency (≥70%), it is necessary to ensure at least the SA decomposition catalyst bed temperature above 750 ℃.
The catalytic activity of Pt/TiO2-850 with different particle sizes. In addition to feeding concentration and temperature, catalyst particle size is also an important factor affecting conversion efficiency. In the previous experiments, the catalyst particle sizes of 0.25–0.425 mm (40–60 mesh) were used. In order to investigate the effect of particle size on the conversion efficiency, three different Pt/TiO2-850 catalyst particles were prepared in the experiment, their particle sizes were respectively 0.425–0.85 mm (20–40 mesh), 0.85–2 mm (10–20 mesh) and 2–3.35 mm (6–10 mesh). The conversion efficiencies of the catalysts with different particle sizes at different WHSV were experimentally investigated, and the results are shown in Fig. 3(d). With the particle size increasing, the conversion efficiency of Pt/TiO2-850 decreased gradually, and the conversion efficiency of 0.25–0.425 mm particles was the highest. The larger the catalyst particle size, the lower the stacking density of the catalyst bed. Part of the SO3 gas passed through the catalyst bed without decomposition, resulting in lower decomposition efficiency. However, if the particle size of the catalyst is too small, the pressure drop of the catalyst bed would be too large, which would increase the operating cost of the equipment and be detrimental to the subsequent scale-up of the process. Therefore, 0.25–0.425 mm was finally chosen as the catalyst particle diameter used for the reaction.
(a) The conversion efficiency of scaled-up Pt/TiO2-850 at different WHSV. (b) The conversion efficiency of scaled-up Pt/TiO2-850 at different temperatures. (c) The schematic diagram of bayonet tube SA reactor. (d) SA decomposition conversion rate, instantaneous O2 production flow and cumulative production in 100 h. (e) The XRD spectra of Pt/TiO2-850 before and after 100 h reaction. (f) i The fresh Pt/TiO2-850, ii The reacted Pt/TiO2-850. (g) i The SEM image of fresh Pt/TiO2-850, ii The SEM image of reacted Pt/TiO2-850.
3.3 Scale-Up Production and Stability Study of Pt/TiO2-850
Previous studies have tested the SA decomposition efficiency of Pt/TiO2-850 catalysts under different reaction conditions and confirmed that Pt/TiO2-850 catalysts have excellent catalytic performance. However, in order to apply the catalyst to real industrial production, it is equally important that the catalysts can be produced in batch scale-up and have good stability in addition to having excellent catalytic performance. Based on this, in this study, Pt/TiO2-850 catalysts were batch-scaled up to obtain catalysts in the order of kilograms. The scale-up produced catalysts were tested for SA decomposition experiments under different WHSV and temperature, and the results were compared with the catalysts prepared in the laboratory, and the comparison results are shown in Fig. 4(a) and (b). The scaled-up produced Pt/TiO2-850 catalysts also have high conversion efficiency, and the scaled-up production process does not have a great impact on the reaction performance.
The above researches confirmed the good reaction performance of the scaled-up produced Pt/TiO2-850 catalyst, and then the stability of Pt/TiO2-850 catalyst was explored. The stability test was carried out on the IS-100 device previously built by the research group. The SA decomposition reactor used a bayonet-tube reactor, and its structure is shown in Fig. 4(c). Compared with general fixed-bed reactors, the bayonet-tube reactor enables heat recovery of the reaction products and improve the process heat utilization efficiency. The material of the reactor is SiC, an industrial material with high hardness, high heat transfer coefficient, and resistance to SA corrosion [34, 35]. Previous simulation studies have shown that bayonet-tube reactor can better utilize heat and improve the conversion efficiency of SA decomposition reactions [29]. A 100 h SA decomposition experiment was carried out in the bayonet tube SiC reactor to test the stability of the Pt/TiO2-850 catalyst. The instantaneous and cumulative flow rates of O2 were recorded by a mass flow meter during the reaction. The results are shown in Fig. 4(d). The reaction produced 5726 L O2 for 100 h, which is higher than the initial target of 5000 L. The average conversion rate of the Pt/TiO2-850 catalyst was 82.6%, and the O2 instantaneous flow rate was maintained at about 0.95 L/min. The initial conversion efficiency of Pt/TiO2-850 reached 87.7%, which was higher than that of the quartz fixed-bed reactor. Although the conversion efficiency of the catalyst decreased with increasing reaction time, the catalytic performance showed a stable trend in the later stage of the reaction, and the conversion efficiency of the catalyst remained stable at about 78.6% at the end of the reaction. Compared with the Pt/SiC, CuCr2O4, and CuFe2O4 SA decomposition catalysts previously studied in the laboratory, Pt/TiO2-850 showed good stability. Combined with its excellent catalytic performance and easy scale-up production, Pt/TiO2-850 can be used as a SA decomposition catalyst for the pilot scale-up of the IS cycle.
The SA decomposition reaction is in a high-temperature, strongly acidic environment, which is a great challenge to the stability of the catalyst. The Pt/TiO2-850 catalyst still maintains high catalytic performance after 100 h of high-temperature SA decomposition reaction. Investigating the structural changes of Pt/TiO2-850 catalysts before and after the reaction and understanding their conformational relationships are important guidelines for the design and development of high-performance and low-cost industrial catalysts for SA decomposition. Based on this, the specific surface area and metal Pt content of the SA decomposition catalysts were tested after 100 h of reaction in this study. After 100 h of reaction, the specific surface area of Pt/TiO2-850 catalyst was 8.333 m2/g, and the Pt content was 5650 μg/g. Compared with that before the reaction, the specific surface area and Pt content of the catalyst decreased. To further investigate the changes of Pt/TiO2-850 catalyst before and after the reaction, XRD and SEM characterization were performed and the results were compared with the fresh catalysts as shown in Fig. 4(e)–(g). After a 100 h high-temperature SA decomposition reaction, the carrier of the catalyst changed from the anatase phase to the rutile phase. Macroscopically, the catalyst changed from gray particles to yellow-brown particles without any obvious change in particle size. Microscopically, the catalyst was agglomerated from uniform nanoparticle size to larger irregularly shaped particles, which indicated that the catalyst had appeared “sintering” phenomenon, and this result was consistent with the specific surface area. Although the “sintering” of the catalyst occurred after the reaction, the conversion efficiency of Pt/TiO2-850 did not decrease significantly. The stability of Pt/TiO2 is better than that of other Pt-loaded catalysts with carriers such as Al2O3, SiC, SiO2, and BaSO4. The reasons for this could be: (1) TiO2 has good acid resistance and would not be dissolved by SA; (2) Pt and TiO2 have strong metal-carrier interactions at high temperatures, and the carrier TiO2 would encapsulate the Pt nanoparticles to prevent Pt loss and “sintering” [36]. These factors ensure the stability of Pt/TiO2. In addition, the comparison of the catalysts before and after the reaction shows that the change in the crystalline structure of the carrier and the decrease in the specific surface area and Pt content during the reaction do not cause the deactivation of the Pt/TiO2-850 catalyst, which provides a direction for the subsequent Pt-loaded SA decomposition catalysts.
4 Conclusion
In this study, a series of SA decomposition catalysts were prepared using anatase TiO2 and Ta2O5 as carriers and precious metal Pt as the active component. All catalysts exhibited SA decomposition catalytic performance at 850 ℃. Among the many catalysts, Pt/TiO2-850 catalyst had the highest reactivity and its conversion efficiency reached up to 84%. It was demonstrated that the Pt/TiO2-850 catalysts had good catalytic activity under different reaction conditions. Furthermore, the scaled-up production of Pt/TiO2-850 catalysts does not affect the catalytic performance. The Pt/TiO2-850 catalyst was tested in a bayonet-tubular SiC reactor with a high throughput lifetime of 100 h. Its conversion efficiency still reached 78.6% at the end of the reaction, which proved that the Pt/TiO2-850 catalyst has good stability. This work researched a SA decomposition catalyst with high reaction performance, easy to scale-up production, and good stability, which laid a solid foundation for the industrial application of SA decomposition catalyst.
References
Kovač, A., Paranos, M., Marciuš, D.: Hydrogen in energy transition: a review. Int. J. Hydrogen Energy 46, 10016–10035 (2021)
Okolie, J.A., Patra, B.R., Mukherjee, A., Nanda, S., Dalai, A.K., Kozinski, J.A.: Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy. Int. J. Hydrogen Energy 46, 8885–8905 (2021)
Hou, P., Zhou, Y., Guo, W., Ren, P., Guo, Q., Xiang, H., et al.: Rational design of hydrogen-donor solvents for direct coal liquefaction. Energy Fuels 32, 4715–4723 (2018)
Fan, L., Tu, Z., Chan, S.H.: Recent development of hydrogen and fuel cell technologies: a review. Energy Rep. 7, 8421–8446 (2021)
Ping, Z., Laijun, W., Songzhe, C., Jingming, X.: Progress of nuclear hydrogen production through the iodine–sulfur process in China. Renew. Sustain. Energy Rev. 81, 1802–1812 (2018)
Zhang, P., Xu, J., Shi, L., Zhang, Z.: Nuclear hydrogen production based on high temperature gas cooled reactor in China. Strateg. Study Chinese Acad. Eng. 21, 20–28 (2019)
Gabriel, K.S., El-Emam, R.S., Zamfirescu, C.: Technoeconomics of large-scale clean hydrogen production—a review. Int. J. Hydrogen Energy (2021)
Pinsky, R., Sabharwall, P., Hartvigsen, J., O’Brien, J.: Comparative review of hydrogen production technologies for nuclear hybrid energy systems. Prog. Nucl. EnergyNucl. Energy 123, 103317 (2020)
Dehghani, S., Sayyaadi, H.: Energy and exergetic evaluations of Bunsen section of the sulfur–iodine thermochemical hydrogen production plant. Int. J. Hydrogen Energy 38, 9074–9084 (2013)
Huang, C., Ali, T.: Analysis of sulfur–iodine thermochemical cycle for solar hydrogen production. Part I: decomposition of sulfuric acid. Sol. Energy 78, 632–646 (2005)
Tebibel, H., Medjebour, R.: Comparative performance analysis of a grid connected PV system for hydrogen production using PEM water, methanol and hybrid sulfur electrolysis. Int. J. Hydrogen Energy 43, 3482–3498 (2018)
Beghi, G.: A decade of research on thermochemical hydrogen at the Joint Research Centre-Ispra. Hydrogen Systems, pp. 153–171. Elsevier (1986)
Pen, M., Gomez, J., Fierro, J.L.G.: New catalytic routes for syngas and hydrogen production. Appl. Catal. A: Gen. 144, 7–57 (1996)
Rashkeev, S.N., Ginosar, D.M., Petkovic, L.M., Farrell, H.H.: Catalytic activity of supported metal particles for sulfuric acid decomposition reaction. Catal. Today. Today 139, 291–298 (2009)
Ishikawa, H., Ishii, E., Uehara, I., Nakane, M.: Catalyzed thermal decomposition of H2SO4 and production of HBr by the reaction of SO2 with Br2 and H2O. Int. J. Hydrogen Energy 7, 237–246 (1982)
Kim, T.H., Gong, G.T., Lee, B.G., Lee, K.Y., Jeon, H.Y., Shin, C.H., et al.: Catalytic decomposition of sulfur trioxide on the binary metal oxide catalysts of Fe/Al and Fe/Ti. Appl. Catal. ACatal. A 305, 39–45 (2006)
Ping, Z., Bo, Y., Lei, Z.: Mechanism of oxygen releasing of copper ferrite in the formation of the corresponding oxygen-deficient compound. Sci. China Chem. 101 (2009)
Bo, Y.U., Zhang, P., Zhang, L., Chen, J., Xu, J.: Studies on the preparation of active oxygen-deficient copper ferrite and its application for hydrogen production through thermal chemical water splitting. 中国科学: 化学英文版 9 (2008)
Banerjee, A.M., Pai, M.R., Bhattacharya, K., Tripathi, A.K., Kamble, V.S., Bharadwaj, S.R., et al.: Catalytic decomposition of sulfuric acid on mixed Cr/Fe oxide samples and its application in sulfur–iodine cycle for hydrogen production. Int. J. Hydrogen Energy 33, 319–326 (2008)
Abimanyu, H., Jung, K.D., Jun, K.W., Kim, J., Yoo, K.S.: Preparation and characterization of Fe/Cu/Al2O3-composite granules for SO3 decomposition to assist hydrogen production. Appl. Catal. ACatal. A 343, 134–141 (2008)
Banerjee, A., Pai, M., Tewari, R., Raje, N., Tripathi, A., Bharadwaj, S., et al.: A comprehensive study on Pt/Al2O3 granular catalyst used for sulfuric acid decomposition step in sulfur–iodine thermochemical cycle: changes in catalyst structure, morphology and metal-support interaction. Appl. Catal. BCatal. B 162, 327–337 (2015)
Nagaraja, B.M., Jung, K.D., Ahn, B.S., Abimanyu, H., Yoo, K.S.: Catalytic decomposition of SO3 over Pt/BaSO4 materials in sulfur−iodine cycle for hydrogen production. Ind. Eng. Chem. Res. 48, 1451–1457 (2009)
Lee, S.Y., Jung, H., Kim, W.J., Shul, Y.G., Jung, K.-D.: Sulfuric acid decomposition on Pt/SiC-coated-alumina catalysts for SI cycle hydrogen production. Int. J. Hydrogen Energy 38, 6205–6209 (2013)
Noh, S.-C., Lee, S.Y., Shul, Y.G., Jung, K.-D.: Sulfuric acid decomposition on the Pt/n-SiC catalyst for SI cycle to produce hydrogen. Int. J. Hydrogen Energy 39, 4181–4188 (2014)
Petkovic, L., Ginosar, D., Rollins, H., Burch, K., Pinhero, P., Farrell, H.: Pt/TiO2 (rutile) catalysts for sulfuric acid decomposition in sulfur-based thermochemical water-splitting cycles. Appl. Catal. ACatal. A 338, 27–36 (2008)
Nur, A.S., Matsukawa, T., Hinokuma, S., Machida, M.: Catalytic SO3 decomposition activity and stability of Pt supported on anatase TiO2 for solar thermochemical water-splitting cycles. ACS Omega 2, 7057–7065 (2017)
Nur, A.S., Matsukawa, T., Funada, E., Hinokuma, S., Machida, M.: Platinum supported on Ta2O5 as a stable SO3 decomposition catalyst for solar thermochemical water splitting cycles. ACS Appl. Energy Mater. 1, 744–750 (2018)
Gao, Q., Sun, Q., Zhang, P., Peng, W., Chen, S.: Sulfuric acid decomposition in the iodine–sulfur cycle using heat from a very high temperature gas-cooled reactor. Int. J. Hydrogen Energy 46, 28969–28979 (2021)
Sun, Q., Gao, Q., Zhang, P., Peng, W., Chen, S.: Modeling sulfuric acid decomposition in a bayonet heat exchanger in the iodine-sulfur cycle for hydrogen production. Appl. Energy 277, 115611 (2020)
Sun, Q., Gao, Q., Zhang, P., Peng, W., Chen, S., Zhao, G., et al.: Numerical study of heat transfer and sulfuric acid decomposition in the process of hydrogen production. Int. J. Energy Res. 43, 5969–5982 (2019)
Kim, S.S., Hong, S.C.: Relationship between the surface characteristics of Pt catalyst and catalytic performance on the H2 SCR. J. Ind. Eng. Chem. 16, 992–996 (2010)
Somorjai, G.A., Li, Y.: Introduction to Surface Chemistry and Catalysis. Wiley (2010)
Schwartz, D., Gadiou, R., Brilhac, J.-F., Prado, G., Martinez, G.: A kinetic study of the decomposition of spent sulfuric acids at high temperature. Ind. Eng. Chem. Res. 39, 2183–2189 (2000)
Takeuchi, Y., Park, C., Noborio, K., Yamamoto, Y., Konishi, S.: Heat transfer in SiC compact heat exchanger. Fusion Eng. Des. 85, 1266–1270 (2010)
Wang, Z., Liu, Y., Zhang, H., Jiang, J., Lin, T., Liu, X., et al.: Joining of SiC ceramics using the Ni-Mo filler alloy for heat exchanger applications. J. Eur. Ceram. Soc. 41, 7533–7542 (2021)
Khan, H.A., Kim, S., Jung, K.-D.: Origin of high stability of Pt/anatase-TiO2 catalyst in sulfuric acid decomposition for SI cycle to produce hydrogen. Catal. Today. Today 352, 316–322 (2020)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this paper
Cite this paper
Zhang, P., Chen, S., Wang, L., Zhang, P. (2024). Study on the High-Performance Catalyst for Sulfuric Acid Decomposition in the IS Cycle. In: Sun, H., Pei, W., Dong, Y., Yu, H., You, S. (eds) Proceedings of the 10th Hydrogen Technology Convention, Volume 1. WHTC 2023. Springer Proceedings in Physics, vol 393. Springer, Singapore. https://doi.org/10.1007/978-981-99-8631-6_36
Download citation
DOI: https://doi.org/10.1007/978-981-99-8631-6_36
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8630-9
Online ISBN: 978-981-99-8631-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)