Abstract
Optimizing flow field structure can effectively improve the performance of Proton Exchange Membrane Fuel Cell (PEMFC). Adding the block in the flow channel is one of the approaches. In this work, the enhanced transport characteristic of the block is re-examined, and its effect on the performance of the fuel cell scale-up is further revealed. The models of single-channel fuel cells with different lengths L and blockage ratio β are developed. Results show that the best cell performance exhibits when β = 100% due to the combined effect of the block and upstream zone. The convection appears below the block, and higher upstream pressure is induced, both of which increase the oxygen concentration at the catalyst layer. Besides, results indicate that the performance of the scaled-up fuel cell with blockage increases at a slower rate. Combined with the pump power, it is found that the addition of the block with β = 100% is indeed beneficial for the fuel cell scale-up. The findings of different blockage effects on different-sized cells provide guidelines for the flow field design.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Proton Exchange Membrane Fuel Cell (PEMFC) has a wide range of application prospects in stationary power plants, transportation, and other industries with the benefits of high power density and efficiency, no carbon emissions, moderate operating temperature, etc. [1, 2]. However, development bottlenecks exist in the commercialization of PEMFC, primarily in three technical indicators of performance, cost, and durability [3]. Optimizing flow field structure can effectively improve the performance of PEMFC.
The flow channel structure of Bipolar Plate (BP) has been extensively investigated throughout the years. The two most prevalent flow channel types could be classified as fine channel-type and pin-type [4]. In addition to the traditional parallel, serpentine, and interdigitated flow channel, the fine channel-type flow channel also comprises the flow channel with blocks (or baffles) and the bionic flow channel, etc. In the pin-type flow channel, a set of regularly spaced obstacles protrudes from the flow field plate. The reactant gas flows in the flow channel formed by the set of obstacles, with the main flow characteristic being the bypass flow in the confined space rather than the inward flow in the fine channel. Benefits and drawbacks of the conventional flow channel are outlined in the literature [5,6,7]. The fine channel-type flow channel structure is the most frequently used in practice, notably the parallel flow channel, which is the most typical.
In the past decade, the modified version of parallel flow channels has been widely studied [8]. Adding blocks (or baffles) in the flow channel could effectively improve the transport of reactants to the porous electrode and thus improve the fuel cell performance. Li et al. [9] optimized the design of the flow channel with partially blocked blocks, and the results showed that when the number of blocks was 16, the net power density of the fuel cell reached the maximum. Ghanbarian et al. [10] considered the effect of various shapes of blocks (square, semicircular and trapezoidal) in the cathode flow channel on the performance of PEMFC. The staggered case outperformed the in-line case by 18% when Heidary et al. [11] compared the results of in-line and staggered configurations of blocks in the parallel flow channel. Chen et al. [12] investigated the impact of the backwind length of the baffle on the output power and the pressure loss.
Single channel is the most basic type of flow channel. Wu et al. [13] inserted different numbers of cuboid blocks laterally on the flow channel and found that the performance of the fuel cell was better under a reasonable pressure drop. Heidary et al. [14] numerically studied the effect of partially blocked and entirely blocked blocks on the performance of the fuel cell. Wang et al. [15] established a 3D multiphase fuel cell model with the Forchheimer inertial effect to simulate the baffle-induced convection more accurately. The results of Perng et al. [16] showed that the trapezoidal baffle with an angle of 60° and a height of 1.125 mm improved the net power the best among all designs of the trapezoidal baffle by about 90% compared to the conventional straight flow channel without baffles. Fan et al. [17] studied baffles of multi-plate structure and integrated structure using a 3D multiphase numerical model. Chen et al. [18] proposed a streamlined porous baffle-oriented flow channel to numerically investigate the performance of the fuel cell and water distribution in the straight flow channel. In contrast to the conventional straight flow channel, Guo et al. [19] demonstrated that both the single-block and the double-block flow channel could facilitate the removal of liquid water.
In conclusion, the placement of blocks in the flow channel could effectively enhance the transport of reactants from the flow channel to the gas diffusion layer, thus improving the performance of PEMFC. However, most of the studies focused on the enhanced transport characteristic in the block zone, while the effect of upstream pressure was not elucidated. In addition, the scale-up is a requirement for the commercialization of PEMFC. The study of the role of the block during the fuel cell scale-up is lacking.
Therefore, a systematic investigation is conducted for single-channel fuel cells with different lengths L and blockage β based on CFD software Fluent. The enhanced transport characteristic of the block is re-examined, and its effect on the performance of the fuel cell scale-up is further revealed.
2 Modeling and Simulation
2.1 Physic Model
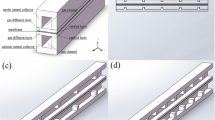
A rectangular block is set inside the cathode flow channel (the width of the block is equal to the width of the flow channel), and the PEMFC model is established with different values of β (the height ratio of the block to the flow channel \(\beta = \frac{{h_{{{\text{block}}}} }}{h}\), , i.e., the blockage ratio) and lengths L (25 mm, 50 mm, 100 mm, 150 mm, and 200 mm).
In Fig. 1, the computational domain and mesh of 25 mm PEMFC are displayed. Bipolar Plate (BP), Gas Diffusion Layer (GDL), Catalyst Layer (CL), and Proton Exchange Membrane (PEM) are all included in the computational domain. Hexahedron mesh is used in this model. The mesh for GDL, CL PEM is denser.
Geometric parameters of the PEMFC model are shown in Table 1. Operating parameters are adjusted according to the experiment of Wang et al. [20] in Sect. 2.6, and are shown in Table 2.
2.2 Numerical Model
Assumptions
For the convenience of computation, the following assumptions are made for the numerical model developed:
-
(1)
The fuel cell operates under the steady-state condition.
-
(2)
Raw material and product water are limited to the gas phase, and there is no phase transition during the operation.
-
(3)
All gases are incompressible ideal gases and follow the ideal gas equation.
-
(4)
The flow of fluid during the operation is laminar flow (Re < 2000).
-
(5)
The isotropic and uniform porous media structure in Membrane Electrode Assembly (MEA).
Governing Equations
Due to the constant flow and incompressibility assumptions, the mass conservation equation is
where ε is the porosity of the porous media layer, ε = 1 in the flow channel; ρ is the density; \(\vec{u}\) is the fluid velocity; Sm is the mass source term, which is solved separately according to different computational domains. Sm = 0 for the flow channel and GDL; Sm for the anode and cathode CL is expressed by the following equation
where M, F, and i are the molar mass, Faraday constant and the exchange current density, respectively. Subscripts a and c denote the anode and the cathode, respectively.
Momentum conservation equation is
where p, μ, and Su are the pressure, the viscosity, and the momentum source term, respectively. In the flow channel, Su = 0; According to Darcy’s law, in the porous media
where κ is the permeability.
Energy conservation equation is
where T, cp, and keff denote the temperature, the heat capacity at constant pressure, and the effective thermal conductivity, respectively. Due to the irreversible process during the operation, not all chemical energy is utilized to provide the electrical work. The remaining energy conversion is incorporated into the source term Sh. Sh remains constant during the reaction process since there is no phase change. Sh is expressed by the following equation
In this equation, the right three terms are the net enthalpy change of the reaction, the heat generated by the overpotential, and the ohmic heat generated by the conduction medium, respectively.
Species conservation equation is
where ci, \(\vec{J}_{{\text{i}}}\), and Si denote the species concentration, the vector of diffusion flux, and the species source term, respectively. The subscript i indicates species (at the anode indicates H2 and H2O, respectively and at the cathode indicates O2, N2, and H2O, respectively). \(\vec{J}_{{\text{i}}}\) Can be obtained from the Stefan-Maxwell equation [21]
where \(D_{{{\text{ij}}}}^{{{\text{eff}}}}\) is the effective diffusion coefficient based on the binary species i-j, whose expression is [22]
where the binary diffusion coefficient Dij is expressed by[21]
where M is the molar mass and vi is the diffusion volume of different gas species molecules.
The species source term Si in Eq. (7) is 0 in the flow channel and GDL. In CL, the source terms for H2, O2, and H2O are
The core of electrochemical reaction model is the computation of electrochemical reaction rate, which is described using Butler-Volmer equation
The above two equations consider the effects of the reactant concentration, temperature, and overpotential on the electrochemical reaction. Where η is the overpotential; ζ is the specific active surface area; iref is the reference exchange current density; ci, ci,ref are the local molar concentration and the reference molar concentration of species i, respectively; α is the charge transfer coefficient.
The driving force of the electrochemical reaction is the difference between the potential of the solid phase and the potential of the membrane phase, both of which are called bipotential. On this basis, the corresponding two charge conservation equations are proposed for electron conduction in solid conducting materials (solid regions of Current Collector and porous layers) and proton transfer in the proton exchange membrane electrolyte in the form of
where Φsol and Φpem are the solid phase potential and the membrane phase potential, respectively; isol and ipem are the electron current and the proton current source term, respectively, and isol + ipem = 0; σ is the conductivity of the solid phase membrane phase. Current source terms are somewhat different at the anode and the cathode. At anode, isol = − ia < 0 and ipem = ic > 0; at cathode, isol = ia > 0 and ipem = − ic < 0.
The relationship between the overpotential of the anode and the cathode, bipotential, and electrode potential is
For the model of water transfer in PEM, the proton conductivity σpem in Eq. (15) can be expressed as
where λ is the number of water molecules per sulfonic acid group, and the relationship between it and the water saturation α is
where the activity of water a is expressed by
where pwv is the pressure of the water vapor; psat is the saturation vapor pressure.
The mode of water transfer in the membrane includes electromigration and differential concentration diffusion. The electromigration water flux is
where \(n_{{\text{d}}} = \frac{2.5\lambda }{{22}}\),
nd is the electromigration coefficient, i.e., the number of water molecules carried per transferred proton.
The differential diffusion water flux in PEM is
where ρm and Mm are the density and the equivalent mass of the membrane, respectively. The diffusion coefficient of water in the membrane Dl is expressed by
where
2.3 Boundary Conditions
The inlet mass flow rate is set to 1.5 × 10–7 kg/s at the anode and 1.25 × 10–6 kg/s at the cathode for the 25 mm PEMFC, both of which increase proportionally with the length of the fuel cell.
The boundary conditions required for the computational domain are shown in Table 3. In addition, the no-slip boundary condition is set for all solid surfaces, and the isothermal condition is set for all walls.
2.4 Numerical Solution Method
The above control equations are solved using CFD software Fluent. In the numerical simulation, all differential equations are discretized by the finite volume method. The Semi-Implicit Method for Pressure Linked Equations (SIMPLE) algorithm for the pressure-velocity coupling is employed. Based on the constant pressure solution, the cathode voltage is set to a specific potential. The value starts at a voltage near the open-circuit voltage and gradually decreases, with each solution converging.
2.5 Mesh Independence Test
To avoid the influence of mesh numbers on simulation results, the mesh independence test is required. The total mesh numbers are set to 98,400, 135,000, 390,000, and 765,000, respectively. Polarization curves are made for each setting of mesh numbers, as shown in Fig. 2. The current density at 0.3 V increases gradually as mesh numbers increase, with current density growth rates of 1.12%, 1.84%, and 0.50%, respectively. It can be seen that the growth rate of current density decreases when the mesh numbers increase from 390,000 to 765,000. The current densities are essentially comparable at these two settings of mesh numbers. Considering the computational resource comprehensively, a setting with mesh numbers of 390,000 is employed for the follow-up work.
2.6 Model Validation
To validate the accuracy of the model, the polarization curve of the single-channel fuel cell obtained from the numerical simulation is compared with experimental values by varying corresponding simulation parameters according to the experimental conditions of Wang et al. [20]. The experiment of Wang et al. [20] was also used to validate the model in other simulation studies [23,24,25]. The comparison result is given in Fig. 3. It can be seen that numerical results are in good agreement with experimental data.
Comparison of numerical results with experiment data from [20].
3 Results and Discussion
3.1 Effect of the Block on the Performance of PEMFC
Comparison of the Electrochemical Performance
A block is placed in the cathode flow channel for fuel cells with different lengths (25 mm, 50 mm, 100 mm, 150 mm, 200 mm). The distance from the block to the cathode inlet equals the distance from the block to the cathode outlet (lc-u = lc-d). Simulations are performed without the block (β = 0%) and with β = 50%, 90%, and 100%, respectively. Corresponding polarization curves are made (as shown in Fig. 4).
The polarization curves at β = 50% and β = 0% largely overlap, indicating that the block with β = 50% has almost no effect on the performance. The polarization curve shifts at β = 90%, and the performance is improved. While at β = 100%, the performance improvement is more obvious. Taking the 25 mm fuel cell as an example, the power density at 0.5 V operating voltage increases by 0.37% at β = 50%, 1.30% at β = 90%, and 13.26% at β = 100% compared to the case without the block.
Analysis for the Enhanced Transport Characteristic
Since the block with β = 100% has the best effect on the performance enhancement, the enhanced transport characteristic of this block setting is analyzed for the 25 mm fuel cell as an example. 0.5 V operating voltage is selected. The fuel cell is divided into three regions (as shown in Fig. 5): Upstream (0–9.5 mm), Block Zone (9.5–15.5 mm), and Downstream (15.5–25 mm). The division is based on the convection range (Section 0) of Block Zone.
The oxygen molar concentration distribution at the cathode GDL / CL interface (as shown in Fig. 6) shows that the oxygen concentration in the block zone and Upstream increases significantly after the addition of the block with β = 100%, indicating that this block setting enhances the mass transfer of oxygen in the above two regions.
Figure 7 shows the oxygen mass flow reduction rate in each region compared with the previous region (the previous region of the upstream flow channel is the flow channel inlet). After adding the block with β = 100%, the oxygen mass flow reduction rate in the upstream flow channel and the block zone (from 15.35% to 18.97% and from 10.83% to 15.21%, respectively) increases significantly, while in the downstream flow channel remains unchanged (from 17.99% to 17.29%). In general, the action range of the block is in the block zone and the Upstream. Next, the enhanced transport characteristics of the block with β = 100% in the block zone and Upstream will be analyzed in detail, respectively.
Effect of the Block Zone
Figure 8 shows that the block with β = 100% completely blocks the flow channel, the reactant gas is forced to bypass the block and enter GDL. The mass transfer in the block zone is enhanced.
Effect of the Upstream
Figure 9 presents the pressure in the cathode flow channel, GDL, and CL. The upstream pressure at β = 90% increases somewhat compared with that at β = 0% and 50%, but the increase is insignificant. While the block with β = 100% significantly increases the pressure in the upstream flow channel, GDL, and CL, and the maximum pressure in each region increases from 101,391.94 Pa, 101,374.27 Pa, and 101,373.38 Pa without the block to 187,249.47 Pa, 187,227.55 Pa, and 187,225.88 Pa, respectively. The pressure of each downstream component does not differ significantly at different β.
It is also observed that the upstream and downstream GDL and CL show pressure dip and surge regions in the local magnified view at β = 100%, which is because this region is located at the junction of the upstream and downstream with the block zone and is affected by the convective effect of the block.
From the ideal gas equation, it is known that the increase of the pressure will drive the gas concentration to increase. As the increase of the upstream pressure, the oxygen concentration increases corresponding and thus improves the electrochemical performance.
The enhanced transport characteristic of the Upstream on the performance of the fuel cell is further verified by the oxygen molar concentration in the cathode flow channel, GDL, and CL (as shown in Fig. 10). At β = 100%, the oxygen molar concentration in the upstream flow channel, GDL, and CL is significantly higher than that at the other three β. The maximum upstream concentration in the cathode flow channel, GDL, and CL increases from 5.81 mol·m−3, 2.67 mol·m−3, and 2.43 mol·m−3 without the block to 10.72 mol·m−3, 4.33 mol·m−3, and 3.82 mol·m−3, respectively.
In summary, the enhanced transport characteristic of the block on the performance of the fuel cell is reflected in two aspects: on the one hand, the convection forms in the block zone, forcing more gas into GDL and thus into CL to participate in the reaction; on the other hand, higher upstream pressure is induced, the upstream oxygen concentration increases accordingly. Therefore, the electrochemical performance is improved.
It should be noted that since the scale-up is a requirement for the commercialization of PEMFC, the enhanced transport characteristic of the block on the single-channel PEMFC scale-up will be analyzed in Sect. 3.2.
3.2 Effect of the Block on the Performance of the Fuel Cell Scale-Up
Comparison of the Electrochemical Performance
Polarization curves of fuel cells with different lengths at different β are given in Fig. 11. At β = 0% and β = 50%, the scale-up is almost unaffected. At β = 90%, the block acts as a performance enhancement during the fuel cell scale-up. At β = 100%, the block has a better effect during the fuel cell scale-up. At this blockage, the power density of the 200 mm fuel cell at 0.5 V operating voltage is improved by 3.08%, 11.19%, and 17.24% compared with that of the 100 mm, 50 mm, and 25 mm fuel cell, respectively.
Analysis for the Scale-Up
Since the block with β = 100% has the best effect on the improvement of the performance of the fuel cell during the scale-up, this block setting was selected for the subsequent analysis. The operating voltage is chosen to be 0.5 V. The upstream range of the 50 mm, 100 mm, 150 mm, and 200 mm fuel cell is set as (0–22) mm, (0–47) mm, (0–72) mm, and (0–97) mm, respectively; the block zone range is set as (22–28) mm, (47–53) mm, (72–78) mm, and (97–103) mm, respectively; the downstream range is set as (28–50) mm, (53–100) mm, (78–150) mm, and (103–200) mm, respectively.
The distribution of the oxygen molar concentration at the cathode GDL/CL interface with different lengths at β = 0% and β = 100% is shown in Figs. 12 and 13. The 50 mm, 100 mm, 150 mm, and 200 mm fuel cells are scaled by a factor of 0.5, 0.25, 0.167, and 0.125, respectively. It is observed that the distribution of the oxygen molar concentration at the GDL / CL interface is nearly same at β = 0%, indicating that the performance during the fuel cell scale-up is unchanged.
At β = 100%, it is observed that the oxygen molar concentration in the block zone and the Upstream gradually increases with length, indicating that the enhanced transport characteristic of the block during the fuel cell scale-up is also reflected in these two regions (the enhanced transport characteristic of the block has been explained in Sect. 3.1). The inlet mass flow rate increases during the scale-up according to the inlet boundary condition. The block obstructs the oxygen flow path in the flow channel, and it will further increase the upstream pressure and intensify the convective diffusion in the block zone as the flow rate increases.
It should be noted that the reinforcing effect of the block with β = 100% on the performance of the fuel cell gradually weakens (as shown in Fig. 14). The power density of fuel cells with different lengths is 1.085 W/cm2, 1.144 W/cm2, 1.234 W/cm2, 1.252 W/cm2, and 1.272 W/cm2, respectively. The reason for this phenomenon is that the percentage of the block zone gradually decreases during the fuel cell scale-up (from 0.24 at 25 mm to 0.03 at 200 mm), decreasing the percentage of the convection range.
3.3 Net Power Density
The block with β = 100% will increase the upstream pressure and thus the pump power. It is necessary to calculate the net power density.
The net power density is calculated by the following equation [26]
where Pnet represents the net power density of the fuel cell, W/m2; Pcell represents the output power density of the fuel cell, W/m2; Ppump represents the pump power density, W/m2; ξ represents the conversion efficiency of the compressor (70% in this study); pa,in and pc,in represent the pressure of anode inlet and cathode inlet, Pa; va,in and vc,in represent the velocity of anode inlet and cathode inlet, m/s; Aa,in and Ac,in represent the area of anode inlet and cathode inlet, m2; Aact represents the activation area, m2.
Table 4 shows the results of the net power calculation. For any given length of the fuel cell, the pump power increases accordingly as β increases. Regardless of the length, the block with β = 100% makes the net power maximum, indicating that such a setting is effective.
The trend of the net power density during the fuel cell scale-up is extracted in Fig. 15. At β = 0%, the net power density gradually decreases during the scale-up. The net power densities of fuel cells with different lengths are 9577.969 W/m2, 9571.912 W/m2, 9557.906 W/m2, 9543.892 W/m2 and 9520.886 W/m2, respectively. Conversely, at β = 100%, the net power density gradually increases during the scale-up. The net power densities of fuel cells with different lengths are 10126.449 W/m2, 10518.001 W/m2, 11239.185 W/m2, 11317.345 W/m2, and 11438.903 W/m2, respectively. Combined with the studies in Sects. 3.1 and 3.2, the addition of the block with β = 100% is indeed beneficial for the fuel cell scale-up.
4 Conclusion
The models of single-channel fuel cells with different lengths L and blockage ratio β are developed. The enhanced transport characteristic of the block is re-examined, and its effect on the performance of the fuel cell scale-up is further revealed. The way of effective utilization of the block is illustrated. The main conclusions are as follows:
-
(1)
The most significant improvement in the performance of the fuel cell is achieved by the block with β = 100%. The power density increased by 0.37% at β = 50%, by 1.30% at β = 90%, and by 13.26% at β = 100%, respectively, compared with the case without the block for the 25 mm fuel cell. The enhanced transport characteristic of the block with β = 100% is reflected in two aspects: on the one hand, the convection forms in the block zone, forcing more reactant gas into GDL and enhancing the mass transfer; on the other hand, higher upstream pressure is induced, which in turn increases the upstream oxygen concentration at GDL/CL interface and improves the electrochemical performance.
-
(2)
The block with β = 100% enhances the performance of the fuel cell during the scale-up. The power density of the 200 mm fuel cell at 0.5 V operating voltage increased by 3.08%, 11.19%, and 17.24% compared with that of the 100 mm, 50 mm, and 25 mm fuel cell, respectively. The increase of the mass flow rate during the fuel cell scale-up makes the upstream pressure increase and the convective diffusion in the block zone reinforced. It should be noted that the reinforcing effect of the block with β = 100% on the performance of the fuel cell gradually weakens. The reason for this phenomenon is that the percentage of the block zone gradually decreases during the fuel cell scale-up (from 0.24 at 25 mm to 0.03 at 200 mm), decreasing the percentage of the convection range.
-
(3)
Considering the increase of the upstream pressure by adding the block with β = 100%, the net power density calculation is performed. The net power density gradually decreases at β = 0%, while the net power density gradually increases during the scale-up at β = 100%. This indicates that the addition of the block with β = 100% is indeed beneficial for the fuel cell scale-up.
References
Alaswad, A., Dassisti, M., Prescott, T., Olabi, A.G.: Technologies and developments of third generation biofuel production. Renew. Sustain. Energy Rev. 51, 1446–1460 (2015)
Alaswad, A., Baroutaji, A., Achour, H., Carton, J., Al Makky, A., Olabi, A.G.: Developments in fuel cell technologies in the transport sector. Int. J. Hydrogen Energy 41(37), 16499–16508 (2016)
Shahgaldi, S., Hamelin, J.: The effect of low platinum loading on the efficiency of PEMFC’s electrocatalysts supported on TiO2–Nb, and SnO2–Nb: an experimental comparison between active and stable conditions. Energy Convers. Manag. 103, 681–690 (2015)
Pan, W., Wang, P., Chen, X., Wang, F., Dai, G.: Combined effects of flow channel configuration and operating conditions on PEM fuel cell performance. Energy Convers. Manag. 220, 113046 (2020)
Li, X.G., Sabir, M.: Review of bipolar plates in PEM fuel cells: flow-field designs. Int. J. Hydrogen Energy 30(4), 359–371 (2005)
Hamilton, P.J., Pollet, B.G.: Polymer electrolyte membrane fuel cell (PEMFC) flow field plate: design, materials and characterisation. Fuel Cells 10(4), 489–509 (2010)
Liu, H.C., Yang, W.M., Tan, J., An, Y., Cheng, L.S.: Numerical analysis of parallel flow fields improved by micro-distributor in proton exchange membrane fuel cells. Energy Convers. Manag. 176, 99–109 (2018)
Wang, Y., Sun, Z.Y., Yang, L.: Enhancement effects of the obstacle arrangement and gradient height distribution in serpentine flow-field on the performances of a PEMFC. Energy Convers. Manag. 252 (2022)
Li, W.Z., Yang, W.W., Wang, N., Jiao, Y.H., Yang, Y., Qu, Z.G.: Optimization of blocked channel design for a proton exchange membrane fuel cell by coupled genetic algorithm and three-dimensional CFD modeling. Int. J. Hydrogen Energy 45(35), 17759–17770 (2020)
Ghanbarian, A., Kermani, M.J.: Enhancement of PEM fuel cell performance by flow channel indentation. Energy Convers. Manag. 110, 356–366 (2016)
Heidary, H., Kermani, M.J., Advani, S.G., Prasad, A.K.: Experimental investigation of in-line and staggered blockages in parallel flowfield channels of PEM fuel cells. Int. J. Hydrogen Energy 41(16), 6885–6893 (2016)
Chen, H., Guo, H., Ye, F., & Ma, C.F.: An experimental study of cell performance and pressure drop of proton exchange membrane fuel cells with baffled flow channels. J. Power Sources 472 (2020)
Wu, H.W., Ku, H.W.: The optimal parameters estimation for rectangular cylinders installed transversely in the flow channel of PEMFC from a three-dimensional PEMFC model and the Taguchi method. Appl. Energy 88(12), 4879–4890 (2011)
Heidary, H., Kermani, M.J., Dabir, B.: Influences of bipolar plate channel blockages on PEM fuel cell performances. Energy Convers. Manag. 124, 51–60 (2016)
Wang, X. F., Qin, Y. Z., Wu, S. Y., Xiang, S. G., Zhang, J. F., Yin, Y.: Numerical and experimental investigation of baffle plate arrangement on proton exchange membrane fuel cell performance. J. Power Sources 457 (2020)
Perng, S.W., Wu, H.W.: A three-dimensional numerical investigation of trapezoid baffles effect on non-isothermal reactant transport and cell net power in a PEMFC. Appl. Energy 143, 81–95 (2015)
Fan, L., Niu, Z., Zhang, G., Jiao, K.: Optimization design of the cathode flow channel for proton exchange membrane fuel cells. Energy Convers. Manag. 171, 1813–1821 (2018)
Chen, H., Guo, H., Ye, F., Ma, C.F.: A numerical study of orientated-type flow channels with porous-blocked baffles of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 46(57), 29443–29458 (2021)
Guo, Q.Y., Qin, Y.Z.: Numerical investigation of water droplet removal characteristics in novel block channels of PEMFC using dynamic wettability model. Int. J. Hydrogen Energy 46(74), 36890–36902 (2021)
Wang, L., Husar, A., Zhou, T., Liu, H.: A parametric study of PEM fuel cell performances. Int. J. Hydrogen Energy 28(11), 1263–1272 (2003)
Baricci, A., Mereu, R., Messaggi, M., Zago, M., Inzoli, F., Casalegno, A.: Application of computational fluid dynamics to the analysis of geometrical features in PEM fuel cells flow fields with the aid of impedance spectroscopy. Appl. Energy 205, 670–682 (2017)
Berning, T., Djilali, N.: Three-dimensional computational analysis of transport phenomena in a PEM fuel cell—a parametric study. J. Power Sources 124(2), 440–452 (2003)
Kuo, J.K., Yen, T.H., Chen, C.K.: Three-dimensional numerical analysis of PEM fuel cells with straight and wave-like gas flow fields channels. J. Power Sources 177(1), 96–103 (2008)
Nguyen, P.T., Berning, T., Djilali, N.: Computational model of a PEM fuel cell with serpentine gas flow channels. J. Power Sources 130(1), 149–157 (2004)
Hashemi, F., Rowshanzamir, S., Rezakazemi, M.: CFD simulation of PEM fuel cell performance: effect of straight and serpentine flow fields. Math. Comput. Model. 55(3–4), 1540–1557 (2012)
Yong, Z., Shirong, H., Xiaohui, J., Yuntao, Y., Mu, X., Xi, Y.: Characteristics of proton exchange membrane fuel cell considering “dot matrix” gas distribution zones and waveform staggered flow field with cooling channels. Energy Convers. Manag. 267, 115881 (2022)
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this paper
Cite this paper
Wang, B. et al. (2024). Effect of Flow Channel Blockage on the Scale-Up of Proton Exchange Membrane Fuel Cells. In: Sun, H., Pei, W., Dong, Y., Yu, H., You, S. (eds) Proceedings of the 10th Hydrogen Technology Convention, Volume 1. WHTC 2023. Springer Proceedings in Physics, vol 393. Springer, Singapore. https://doi.org/10.1007/978-981-99-8631-6_31
Download citation
DOI: https://doi.org/10.1007/978-981-99-8631-6_31
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8630-9
Online ISBN: 978-981-99-8631-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)