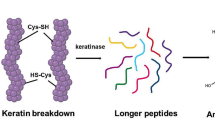
Abstract
The poultry industry is one of the significant driving sectors in the food industry. On one hand, the enormous growth of this industry has boosted food safety. Still, on the other side, it also generates massive amounts of waste during various stages of food processing. Feathers, viscera, bones, and dead on arrival are some of the solid wastes which are generated. The poultry industry’s most abundant wastes include feathers with approximately 90% protein content, mainly keratin protein. Enzyme technology has been one of the solutions for converting these wastes into valuable products, for example, amino acids, peptides, and other bioactive compounds having a physiological role. For this bioconversion, a keratinase enzyme is of utmost importance. Different microbes, bacteria, and fungi can degrade the feathers by secreting keratinase enzyme. This chapter gives an overview of poultry waste management through enzyme keratinase, its structure, different sources of the enzyme, production methods, and the role of the keratinase enzyme in bioconverting poultry waste into valuable products.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abbas A, Mubeen M, Zheng H, Sohail MA, Shakeel Q, Solanki MK, Iftikhar Y, Sharma S, Kashyap BK, Hussain S, del Carmen ZRM, Moya-Elizondo EA, Zhou L (2022) Trichoderma spp. genes involved in the biocontrol activity against Rhizoctonia solani. Front Microbiol 13:1–22. https://doi.org/10.3389/fmicb.2022.884469
Abdel-Fattah AM, El-Gamal MS, Ismail SA, Emran MA, Hashem AM (2018) Biodegradation of feather waste by keratinase produced from newly isolated bacillus licheniformis ALW1. J Genet Eng Biotechnol 16:311–318
Adelere IA, Lateef A (2019) Degradation of keratin biomass by different microorganisms. In: Sharma S, Kumar A (eds) Keratin as a protein biopolymer. Springer series on polymer and composite materials. Springer, Cham, pp 123–162
Adetunji CO, Makanjuola OR, Arowora K, Afolayan S, Adetunji J (2012) Production and application of keratin-based organic fertilizer from microbially hydrolyzed feathers to cowpea (Vigna unguiculata). Int J Eng Sci 3(12):22–29
Anandharaj M, Sivasankari B, Siddharthan N, Rani RP, Sivakumar S (2016) Production, purification, and biochemical characterization of thermostable metalloprotease from novel bacillus alkalitelluris TWI3 isolated from tannery waste. Appl Biochem Biotechnol 178:1666–1686
Arokiyaraj S, Varghese R, Ali Ahmed B, Duraipandiyan V, Al-Dhabi NA (2019) Optimizing the fermentation conditions and enhanced production of keratinase from Bacillus cereus isolated from the halophilic environment. Saudi J Biol Sci 26:378–381
Bagewadi ZK, Mulla SI, Ninnekar HZ (2018) Response surface methodology based optimization of keratinase production from Trichoderma harzianum isolate HZN12 using chicken feather waste and its application in de-hairing of hide. J Environ Chem Eng 6:4828–4839
Balint B, Bagi Z, Toth A, Rakhely G, Perei K, Kovacs KL (2005) Utilization of keratin-containing biowaste to produce biohydrogen. Appl Microbiol Biotechnol 69:404–410
Barone JR, Schmidt WF, Liebner CFE (2005) Thermally processed keratin films. J Appl Polym Sci 97(4):1644–1651
Ben Hamad Bouhamed S, Kechaou N (2017) Kinetic study of sulphuric acid hydrolysis of protein feathers. Bioprocess Biosyst Eng 40(5):715–721
Bhari R, Kaur M, Singh RS, Pandey A, Larroche C (2018) Bioconversion of chicken feathers by Bacillus aerius NSMk2: a potential approach in poultry waste management. Bioresour Technol Rep 3:224–230
Bohacz J (2016) Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World J Microbiol Biotechnol 33:13
Bohacz J, Kornillowicz-Kowalska T (2019) Fungal diversity and keratinolytic activity of fungi from lignocellulosic composts with chicken feathers. Process Biochem 80:119–128
Bragulla HH, Homberger DG (2009) Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat 214:516–559
Brandelli A (2008) Bacterial keratinases: useful enzymes for bioprocessing agro industrial wastes and beyond. Food Bioproc Tech 1:105–116
Brandelli A, Daroit DJ, Riffel A (2010) Biochemical features of microbial keratinases and their production and applications. Appl Microbiol Biotechnol 85:1735–1750
Bressollier P, Letourneau F, Urdaci M, Verneuil B (1999) Purification and characterization of a keratinolytic serine proteinase from Streptomyces albidoflavus. Appl Environ Microbiol 65:2570–2576
Brouta F, Descamps F, Monod M, Vermout S, Losson B, Mignon B (2002) Secreted metalloprotease gene family of Microsporum canis. Infect Immun 70:5676–5683
Calin M, Constantinescu-Aruxandei D, Alexandrescu E, Raut I, Doni MB, Arsene ML (2017) Degradation of keratin substrates by keratinolytic fungi. Electron J Biotechnol 28:101–112
Callegaro K, Welter N, Daroit DJ (2018) Feathers as bioresource: microbial conversion into bioactive protein hydrolysates. Process Biochem 75:1–9
Cavello IA, Hours RA, Rojas NL, Cavalitto SF (2013) Purification and characterization of a keratinolytic serine protease from Purpureocillium lilacinum LPS # 876. Process Biochem 48:972–978
Chaturvedi V, Bhange K, Bhatt R, Verma P (2014) Production of kertinases using chicken feathers as substrate by a novel multifunctional strain of pseudomonas stutzeri and its de-hairing application. Biocatal Agric Biotechnol 3:167–174
Chaya E, Suzuki T, Karita S, Hanya A, Yoshino-Yasuda S, Kitamoto N (2014) Sequence analysis and heterologous expression of the wool cuticle-degrading enzyme encoding genes in fusarium oxysporum 26-1. J Biosci Bioeng 117:711–714
Chen X, Zhou B, Xu M, Huang Z, Jia G, Zhao H, Liu G (2015) Prokaryotic expression and characterization of a keratinolytic protease from aspergillus Niger. Biologia 70(2):157–164
Cheng FY, Wan TC, Huang CW, Tominaga K, Lin LC, Sakata R (2009) The effects of chicken leg bone on antioxidative properties under different heating conditions. Asian Aust J Anim Sci 21:1815–1820
Choinska-Pulit A, Laba W, Rodziewicz A (2019) Enhancement of pig bristles waste bioconversion by inoculum of keratinolytic bacteria during composting. Waste Manag 84:269–276
Darah I, Nur-Diyana A, Nurul-Husna S, Jain K, Lim SH (2013) Microsporum fulvum IBRL SD3: as novel isolate for chicken feathers degradation. Appl Biochem Biotechnol 171:1900–1910
Daroit DJ, Brandelli A (2014) A current assessment on the production of bacterial keratinases. Crit Rev Biotechnol 34(4):372–384
de Guzman RC, Saul JM, Ellenburg MD, Merrill MR, Coan HB, Smith TL (2013) Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 34:1644–1656
Descamps F, Brouta F, Baar D, Losson B, Mignon B (2002) Isolation of a Microsporum canis gene family encoding three subtilisin-like proteases expressed in vivo. J Investig Dermatol 119:830–835
Dias GJ, Mahoney P, Swain M, Kelly RJ, Smith RA, Ali MA (2010) Keratin-hydroxyapatite composites: biocompatibility, osseointegration, and physical properties in an ovine model. J Biomed Mater Res A 95A:1084–1095
Dudynski M, Kwiatkowski K, Bajer K (2012) From feathers to syngas – technologies and devices. Waste Manag 32:685–691
Ebeling W, Hennrich N, Klockow M, Metz H, Orth HD, Lang H (1974) Proteinase K from Tritirachium album limber. Eur J Biochem 47:91–97
Fakhfakh-Zouari N, Haddar A, Hmidet N, Frikha F, Nasri M (2010) Application of statistical experimental design for optimization of keratinases production by Bacillus pumilus A1 grown on chicken feather and some biochemical properties. Process Biochem 45(5):617–626
Fang Z, Zhang J, Liu B, Du G, Chen J (2013) Biodegradation of wool waste and keratinase production in scale-up fermenter with different strategies by Stenotrophomonas maltophilia BBE11-1. Bioresour Technol 140:286–291
Fang Z, Zhang J, Liu B, Jiang L, Du G, Chen J (2014) Cloning, heterologous expression and characterization of two keratinases from Stenotrophomonas maltophilia BBE11-1. Process Biochem 49:647–654
Fellahi S, Chibani A, Feuk-Lagerstedt E, Taherzadeh MJ (2016) Identification of two new keratinolytic proteases from a Bacillus pumilus strain using protein analysis and gene sequencing. AMB Express 6:42–48
Fontoura R, Daroit DJ, Correa APF, Meira SMM, Mosquera M, Brandelli A (2014) Production of feather hydrolysates with antioxidant, angiotensin-I converting enzyme- and dipeptidyl peptidase-IV-inhibitory activities. N Biotechnol 31:506–513
Fraser RDB, Parry DAD (2008) Molecular packing in the feather keratin filament. J Struct Biol 162:1–13
Friedrich AB, Antranikian G (1996) Keratin degradation by Fervidobacterium pennavorans, a novel thermophilic anaerobic species of the order Thermotogales. Appl Environ Microbiol 62:2875–2882
Gegeckas A, Gudiukaite R, Debski J, Citavicius D (2015) Keratinous waste decomposition and peptide production by keratinase from Geobacillus stearothermophilus AD-11. Int J Biol Macromol 75:158–165
Ghosh A, Chakrabarti K, Chattopadhyay D (2009) Cloning of feather-degrading minor extracellular protease from Bacillus cereus DCUW: dissection of the structural domains. Microbiology 155:2049–2057
Gomez-Guillen MC, Gimenez B, Lopez-Caballero ME, Montero MP (2011) Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll 25:1813–1827
Gong J, Wang Y, Zhang D, Li H, Zhang X, Zhang R, Lu Z, Xu Z, Shi J (2015) A surfactant-stable Bacillus pumilus K9 α-keratinase and its potential application in detergent industry. Chem Res Chin Univ 31:91–97
Gonzalo M, Espersen R, Al-Soud WA, Cristiano Falco F, Hagglund P, Sorensen SJ, Svensson B, Jacquiod S (2020) Azo dying of α-keratin material improves microbial keratinase screening and standardization. J Microbial Biotechnol 13(4):984–996
Grazziotin A, Pimentel FA, Sangali S, de Jong EV, Brandelli A (2007) Production of feather protein hydrolysate by keratinolytic bacterium vibrio sp. kr2. Bioresour Technol 98:3172–3175
Gunasekaran J, Kannuchamy N, Kannaiyan S, Chakraborti R, Gudipati V (2015) Protein hydrolysates from shrimp (Metapenaeus dobsoni) head waste: optimization of extraction conditions by response surface methodology. J Aquat Food Prod Technol 24(5):429–442
Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 70:21–33
Gupta S, Singh R (2013) Statistical modeling and optimization of keratinase production from newly isolated Bacillus subtilis RSE163. Int J Adv Biotechnol Res 4:1030–1037
Gupta R, Rajput R, Sharma R, Gupta N (2013) Biotechnological applications and prospective market of microbial keratinases. Appl Microbiol Biotechnol 97:9931–9940
Gurav RG, Jadhav JP (2013) A novel source of biofertilizer from feather biomass for banana cultivation. Environ Sci Pollut Res 20(7):4532–4539
Habbeche A, Saoudi B, Jaouadi B, Haberra S, Kerouaz B, Boudelaa M, Badis A, Ladjama A (2014) Purification and biochemical characterization of a detergent stable keratinase from a newly thermophilic actinomycete Actinomadura keratinilytica strain Cpt29 isolated from poultry compost. J Biosci Bioeng 117:413–421
Hamiche S, Mechri S, Khelouia L, Annane R, El Hattab M, Badis A (2019) Purification and biochemical characterization of two keratinases from bacillus amyloliquefaciens S13 isolated from marine brown alga Zonaria tournefortii with potential keratin-biodegradation and hide-unhairing activities. Int J Biol Macromol 122:758–769
Hassan MA, Abol-Fotouh D, Omer AM, Tamer TM, Abbas E (2020) Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: a review. Int J Biol Macromol 1(154):567–583
Herzog B, Overy DP, Haltli B, Kerr RG (2016) Discovery of keratinases using bacteria isolated from marine environments. Syst Appl Microbiol 39:49–57
Huang S, Liu P (2010) Inhibition of angiotensin I-converting enzymes by enzymatic hydrolysates from chicken blood. J Food Drug Anal 18:458–463
Huang Y, Busk PK, Herbst FA, Lange L (2015) Genome and secretome analyses provide insights into keratin decomposition by novel proteases from the non-pathogenic fungus Onygena corvina. Appl Microbiol Biotechnol 99:9635–9649
Ichida JM, Krizova L, LeFevre CA, Keener HM, Elwell DL, Burtt EH (2001) Bacterial inoculum enhances keratin degradation and biofilm formation in poultry compost. J Microbiol Methods 47:199–208
Jankiewicz U, Larkowska E, Brzezinska MS (2016) Production, characterization, gene cloning, and nematocidal activity of the extracellular protease from Stenotrophomonas maltophilia N4. J Biosci Bioeng 121:614–618
Jany KD, Lederer G, Mayer B (1986) Amino acid sequence of proteinase K from the mold Tritirachium album limber: proteinase K- a subtilisin-related enzyme with disulfide bonds. FEBS Lett 199:139–144
Jayathilakan K, Sultana K, Radhakrishna K, Bawa AS (2012) Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol 49:278–293
Jin HS, Song K, Baek JH, Lee JE, Kim DJ, Nam GW, Kang NJ, Lee DW (2018) Identification of matrix metalloproteinase-1-suppressive peptides in feather keratin hydrolysate. J Agric Food Chem 66:12719–12729
Jousson O, Lechenne B, Bontems O, Mignon B, Reichard U, Barblan J, Quadroni M, Monod M (2004) Secreted subtilisin gene family in Trichophyton rubrum. Gene 339:79–88
Kang E, Jin HS, La JW, Sung JY, Park SY, Kim WC, Lee DW (2020) Identification of keratinases from Fervidobacterium islandicum AW-1 using dynamic gene expression profiling. J Microbial Biotechnol 13:442–457
Kashyap BK, Ara R, Singh A, Kastwar M, Aaysha S, Solanki MK (2019a) Halotolerant PGPR bacteria: amelioration for salinity stress. In: Singh D, Gupta V, Prabha R (eds) Microbial interventions in agriculture and environment. Springer, Singapore, pp 509–530., ISBN 978-981-13-8390-8. https://doi.org/10.1007/978-981-13-8391-5_19
Kashyap BK, Solanki MK, Pandey AK, Prabha S, Kumar P, Kumari B (2019b) Bacillus as plant growth promoting Rhizobacteria (PGPR): a promising green agriculture technology. In: Ansari R, Mahmood I (eds) Plant health under biotic stress. Springer, Singapore, pp 219–236., ISBN: 978-981-13-6040-4. https://doi.org/10.1007/978-981-13-6040-4_11
Khardenavis AA, Kapley A, Purohit HJ (2009) Processing of poultry feathers by alkaline keratin hydrolyzing enzyme from Serratia sp. HPC 1383. Waste Manag 29:1409–1415
Kim WK, Patterson PH (2000) Nutritional value of enzyme - or sodium hydroxide treated feathers from dead hens. Poult Sci 79:528–534
Kim JS, Kluskens LD, De Vos WM, Huber R, Van Der Oost J (2004) Crystal structure of fervidolysin from Fervidobacterium pennivorans, a keratinolytic enzyme related to subtilisin. Acta Sci Biotechnol 335:787–797
Korniłłowicz-Kowalska T, Bohacz J (2011) Biodegradation of keratin waste: theory and practical aspects. Waste Manag 31(8):1689–1701
Kota K, Shaik S, Kota K, Karlapud A (2014) Bioplastic from chicken feather waste. Int J Pharmaceut Sci Rev Res 27(2):373–375
Kshetri P, Roy SS, Sharma SK, Singh TS, Ansari MA, Prakash N (2019) Transforming chicken feather waste into feather protein hydrolysate using a newly isolated multifaceted keratinolytic bacterium Chryseobacterium sediminis RCM-SSR-7. Waste Biomass Valor 10:1–11
Laba W, Choinska A, Rodziewicz A, Piegza M (2015) Keratinolytic abilities of Micrococcus luteus from poultry waste. Braz J Microbiol 46:691–700
Lange L, Huang Y, Busk PK (2016) Microbial decomposition of keratin in nature-a new hypothesis of industrial relevance. Appl Microbiol Biotechnol 100:2083–2096
Langeveld JP, Wang JJ, Van de Wiel DF, Shih GC, Garssen GJ, Bossers A, Shih JC (2003) Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis 188(11):1782–1789
Lasekan A, Bakar A, Hashim D (2013) Potential of chicken by-products as sources of useful biological resources. Waste Manag 33:552–565
Lee YJ, Dhanasingh I, Ahn JS, Jin HS, Choi JM, Lee SH, Lee DW (2015) Biochemical and structural characterization of a keratin-degrading M32 carboxypeptidase from Fervidobacterium islandicum AW-1. Biochem Biophys Res Commun 468:927–933
Li Q (2019) Progress in microbial degradation of feather waste. Front Microbiol 10:2717
Li J, Shi PJ, Han XY, Meng K, Yang PL, Wang YR, Luo HY, Wu NF, Yao B, Fan YL (2007) Functional expression of the keratinolytic serine protease gene sfp2 from Streptomyces fradiae var. k11 in Pichia pastoris. Protein Expr Purif 54:79–86
Li Z, Reimer C, Picard M, Mohanty AK, Misra M (2020) Characterization of chicken feather biocarbon for use in sustainable biocomposites. Front Mater 7:1–12
Liaqat I, Ali S, Butt A, Durrani AI, Zafar U, Saleem S, Naseem S, Ahsan F (2022) Purification and characterization of keratinase from bacillus licheniformis dcs1 for poultry waste processing. J Oleo Sci 71(5):693–700
Liu Q, Long K, Lu F, Chen J (2017) Biodegradation and antibacterial activity of a feather-degrading strain of bacterium. Biocatal Agric Biotechnol 9:195–200
Marquez E, Bracho M, Archile A, Rangel L, Benítez B (2005) Proteins, isoleucine, lysine and methionine content of bovine, porcine and poultry blood and their fractions. Food Chem 93:503–505
Martinez JPDO, Cai G, Nachtschatt M, Navone L, Zhang Z, Robins K, Speight R (2020) Challenges and opportunities in identifying and characterising keratinases for value-added peptide production. Catalysts 10(2):184
Mayne R, Brewton RG (1993) New members of the collagen superfamily. Curr Opin Cell Biol 5:883–890
McKittrick J, Chen PY, Bodde SG, Yang W, Novitskaya EE, Meyers MA (2012) The structure, functions, and mechanical properties of keratin. J Miner Metals Mater Soc 64:449–468
Meyers MA, Chen P, Lin AY, Seki Y (2008) Biological materials: structure and mechanical properties. Prog Mater Sci 53:1–206
Mitsuiki S, Ichikawa M, Oka T, Sakai M, Moriyama Y, Sameshima Y, Goto M, Furukawa K (2004) Molecular characterization of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Enzyme Microb Technol 34:482–489
Monod M, Lechenne B, Jousson O, Grand D, Zaugg C, Stocklin R, Grouzmann E (2005) Aminopeptidases and dipeptidyl-peptidases secreted by the dermatophyte Trichophyton rubrum. Microbiology 151:145–155
Ng CS, Wu P, Fan WL, Yan J, Chen CK et al (2014) Genomic organization, transcriptomic analysis, and functional characterization of avian α- and β-keratins in diverse feather forms. Genome Biol Evol 6:2258–2273
Odetallah NH, Wang JJ, Garlich JD, Shih JCH (2005) Versazyme supplementation of broiler diets improves market growth performance. Poult Sci 84:858–864
Onifade AA, Al-Sane NA, Al-Musallam AA, Al-Zarban S (1998) A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour Technol 66:1–11
Paul T, Halder SK, Das A, Bera S, Maity C, Mandal A et al (2013) Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocatal Agric Biotechnol 2:50–57
Paul T, Das A, Mandal A, Halder SK, Jana A, Maity C, Dasmohapatra PK, Pati BR, Mondal KC (2014) An efficient cloth cleaning properties of a crude keratinase combined with detergent: towards industrial viewpoint. J Clean Prod 66:672–684
Peng Z, Zhang J, Du G, Chen J (2019) Keratin waste recycling based on microbial degradation: mechanisms and prospects. ACS Sustain Chem Eng 7:9727–9736
Qiu J, Wilkens C, Barrett K, Meyer AS (2020) Microbial enzymes catalyzing keratin degradation: classification, structure, function. Biotechnol Adv 44:107607
Qiu J, Barrett K, Wilkens C, Meyer AS (2022) Bioinformatics based discovery of new keratinases in protease family M36. N Biotechnol 68:19–27
Rai S, Solanki MK, Anal AKD, Sagar A, Solanki AC, Kashyap BK, Pandey AK (2020) Emerging Frontiers of microbes as agro-waste recycler. In: Kashyap BK, Solanki MK, Kamboj DV, Pandey AK (eds) Waste to energy: prospects and applications. Springer, Singapore. https://doi.org/10.1007/978-981-33-4347-4_1
Ramakrishna Reddy M, Sathi Reddy K, Ranjita Chouhan Y, Bee H, Reddy G (2017) Effective feather degradation and keratinase production by Bacillus pumilus GRK for its application as bio-detergent additive. Bioresour Technol 243:254–263
Ramnani P, Gupta R (2004) Optimization of medium composition for keratinase production on feather by bacillus licheniformis RG1 using statistical methods involving response surface methodology. Biotechnol Appl Biochem 40:191–196
Riffel A, Brandelli A (2006) Keratinolytic bacteria isolated from feather waste. Braz J Microbiol 37:395–399
Sahni N, Sahota PP, Gupta PU (2015) Bacterial keratinases and their prospective applications: a review. Int J Curr Microbiol App Sci 4:768–783
Salminen E, Rintala J (2002) Anaerobic digestion of organic solid poultry slaughter house waste—a review. Bioresour Technol 83:13–26
Sams AR (2001) Poultry meat processing. CRC, Boca Raton
Saunders SE, Bartelt-Hunt SL, Bartz JC (2008) Prions in the environment: occurrence, fate and mitigation. Prion 2(4):162–169
Schrooyen PMM, Dijkstra PJ, Oberthur RC, Bantjes A, Feijen J (2001) Stabilization of solutions of feather keratins by sodium dodecyl sulfate. J Colloid Interface Sci 240(1):30–39
Sharma R, Gupta R (2010) Extracellular expression of keratinase Ker P from Pseudomonas aeruginosa in E. coli. Biotechnol Lett 32:1863–1868
Sharma A, Chandra S, Sharma M (2012) Difference in keratinase activity of dermatophytes at different environmental conditions is an attribute of adaptation to parasitism. Mycoses 55:410–415
Shavandi A, Silva TH, Bekhit AA, Bekhit AEDA (2017) Keratin: dissolution, extraction and biomedical application. Biomater Sci 5:1699–1735
Shih JCH (1993) Recent development in poultry waste digestion and feather utilization- a review. Poult Sci 72:1617–1620
Shrinivas D, Naik GR (2011) Characterization of alkaline thermostable keratinolytic protease from thermoalkalophilic bacillus halodurans JB 99 exhibiting dehairing activity. Int Biodeter Biodegr 65:29–35
Simpson TW (1991) Agronomic use of poultry industry waste. Poult Sci 70:1126–1131
Solanki MK, Kashyap BK, Solanki AC, Malviya MK, Surapathrudu K (2019) Helpful linkages of Trichodermas in the process of mycoremediation and mycorestoration. In: Ansari R, Mahmood I (eds) Plant health under biotic stress, vol 2. Springer, Singapore. ISBN 978-981-13-8390-8. https://doi.org/10.1007/978-981-13-6040-4_2
Sone T, Haraguchi Y, Kuwahara A, Ose T, Takano M, Abe A, Tanaka M, Tanaka I, Asano K (2015) Structural characterization reveals the keratinolytic activity of an arthrobacter nicotinovorans protease. Protein Pept Lett 22:63–72
Suzuki Y, Tsujimoto Y, Matsui H, Watanabe K (2006) Decomposition of extremely hard-to-degrade animal proteins by thermophilic bacteria. J Biosci Bioeng 102:73–81
Tamreihao K, Devi LJ, Khunjamayum R, Mukherjee S, Ashem RS, Ningthoujam DS (2017) Biofertilizing potential of feather hydrolysate produced by indigenous keratinolytic Amycolatopsis sp. MBRL 40 for rice cultivation under field conditions. Biocatal Agric Biotechnol 10:317–320
Tamreihao K, Mukherjee S, Khunjamayum R, Devi LJ, Asem RS, Ningthoujam DS (2019) Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J Basic Microbiol 59:4–13
Tridico SR, Koch S, Michaud A, Thomson G, Kirkbride KP, Bunce M (2014) Interpreting biological degradative processes acting on mammalian hair in the living and the dead: which ones are taphonomic? Proc R Soc B Biol Sci 281(1796):20141755
Tsiroulnikov K, Rezai H, Bonch-Osmolovskaya E, Nedkov P, Gousterova A, Cueff V, Godfroy A, Barbier G, Métro F, Chobert JM (2004) Hydrolysis of the amyloid prion protein and nonpathogenic meat and bone meal by anaerobic thermophilic prokaryotes and Streptomyces subspecies. J Agric Food Chem 52:6353–6360
Van der Rest M, Garrone R (1991) Collagen family of proteins. FASEB J 5:2814–2823
Vasileva-Tonkova E, Gousterova A, Neshev G (2009) Ecologically safe method for improved feather wastes biodegradation. Int Biodeter Biodegr 63:1008–1012
Verma A, Singh H, Anwar S, Chattopadhyay A, Tiwari KK, Kaur S, Dhilon GS (2017) Microbial keratinases: industrial enzymes with waste management potential. Crit Rev Biotechnol 37:476–491
Verma P, Vasudevan V, Kashyap BK, Samsudeen TI, Meghvansi MK, Singh L, Kamboj DV (2018) Direct lysis glass Milk method of genomic DNA extraction reveals greater archaeal diversity in anaerobic biodigester slurry as assessed through denaturing gradient gel electrophoresis. J Exp Biol Agric Sci 6(2):315–323
Vesela M, Friedrich J (2009) Amino acid and soluble protein cocktail from waste keratin hydrolysed by a fungal keratinase of Paecilomyces marquandii. Biotechnol Bioprocess Eng 14:84–90
Wang L, Cheng G, Ren Y, Dai Z, Zhao ZS, Liu F, Li S, Wei Y, Xiong J, Tang XF (2015) Degradation of intact chicken feathers by Thermoactinomyces sp. CDF and characterization of its keratinolytic protease. Appl Microbiol Biotechnol 99:3949–3959
Wang J, Hao S, Luo T, Cheng Z, Li W, Gao F et al (2017) Feather keratin hydrogel for wound repair: preparation, healing effect and biocompatibility evaluation. Colloids Surf B Biointerfaces 149:341–350
Wu B, Shi P, Li J, Wang Y, Meng K, Bai Y, Luo H, Yang P, Zhou Z, Yao B (2010) A new aminopeptidase from the keratin-degrading strain Streptomyces fradiae var. k11. Appl Biochem Biotechnol 160:730–739
Wu WL, Chen MY, Tu IF, Lin YC, Eswar Kumar N, Chen MY et al (2017) The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci Rep 7:4658
Yang L, Wang H, Lv Y, Bai Y, Luo H, Shi P, Huang H, Yao B (2016) Construction of a rapid feather-degrading bacterium by overexpression of a highly efficient alkaline keratinase in its parent strain bacillus amyloliquefaciens K11. J Agric Food Chem 64:78–84
Yeo I, Lee YJ, Song K, Jin HS, Lee JE, Kim D, Lee DW, Kang NJ (2018) Low molecular weight keratins with anti-skin aging activity produced by anaerobic digestion of poultry feathers with Fervidobacterium islandicum AW-1. J Biotechnol 271:17–25
Yohko Y, Mari M, Mohamed Mahdi A, Michel M, Peter S, Tsuyoshi Y (2014) Flippase (FLP) recombinase-mediated marker recycling in the dermatophyte Arthroderma vanbreuseghemii. Microbiology 160:2122–2135
Yoshioka M, Miwa T, Horii H, Takata M, Yokoyama T, Nishizawa K, Watanabe M, Shinagawa M, Murayama Y (2007) Characterization of a proteolytic enzyme derived from a bacillus strain that effectively degrades prion protein. J Appl Microbiol 102:509–515
Yue XY, Zhang B, Jiang DD, Liu YJ, Niu TG (2011) Separation and purification of a keratinase as pesticide against root-knot nematodes. World J Microbiol Biotechnol 27:2147–2153
Zaugg C, Jousson O, Lechenne B, Staib P, Monod M (2008) Trichophyton rubrum secreted and membrane-associated carboxypeptidases. Int J Med Microbiol 298:669–682
Zhang RX, Gong JS, Dou WF, Zhang DD, Zhang YX, Li H, Lu ZM, Shi JS, Xu ZH (2016) Production and characterization of surfactant-stable fungal keratinase from Gibberella intermedia CA3-1 with application potential in detergent industry. Chem Pap 70:1460–1470
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
The authors have no conflict of interest.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Soni, M., Soni, A., Joshi, C.M., Chhimpa, S., Yadav, J. (2023). Keratinase Role in Management of Poultry Waste. In: Kashyap, B.K., Solanki, M.K. (eds) Current Research Trends and Applications in Waste Management. Springer, Singapore. https://doi.org/10.1007/978-981-99-3106-4_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-3106-4_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-3105-7
Online ISBN: 978-981-99-3106-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)