Abstract
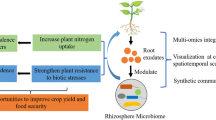
After the green revolution, the consumption of rice and wheat became popular, but grain production became an area of concern. Now, the importance of millet was understood, particularly in developing countries of Asia and Africa. The novel technology termed as ‘metabolomics’ played a major role in improving the quality of crop and enhancing its yield. Metabolomics is the dominant driver of phytochemical analysis in the present times. This tool has helped in finding the quality and quantity of root exudates which determines the phenotypic fate of the cells, tissue, and the plant in whole crop. Metabolomics differs from traditional approaches of phytochemical analysis fundamentally, such as it is based on rational analysis, supported by scientific data, assessing all metabolites that can be measured without any pre-selection. Rhizosphere comprises varied populations wherein the plant roots compete in order to survive. For securing optimum water, essential nutrients and space, plants undergo inter-species competitions, as well as with other microorganisms present in soil. This competition for survival takes place through biochemical interactions in between roots and microbes, and among roots of different plants. Certain root secretions called as ‘root exudates’ are considered to initiate such metabolite communication and command these interactions. These exudates or secondary metabolites play a key role in underground communications between plant roots and beneficial microbes. The ability of plants to communicate simultaneously with other plants and other microbes as well as their potential to alter metabolic processes under stress conditions is an unexplored area. It is of utmost importance to gain more experimental evidence and conduct studies to fully understand the process of underground communication under biotic and abiotic stress conditions as well as nutritional benefits of millets, to improve its quality and production, in order to promote sustainable agriculture and meet the ever-growing demand of food, especially in developing countries like India. Here, we have given an insight into the biochemical interaction between millet plants, studied using a holistic, data-driven approach, termed as metabolomics.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abdul Malik NA, Kumar IS, Nadarajah K (2020) Elicitor and receptor molecules: orchestrators of plant defence and immunity. Int J Mol Sci 21(3):963
Aldon D, Mbengue M, Mazars C, Galaud JP (2018) Calcium signalling in plant biotic interactions. Int J Mol Sci 19(3):665
Amadou I, Amza T, Shi YH, Le GW (2011) Chemical analysis and antioxidant properties of foxtail millet bran extracts. Songklanakarin J Sci Technol 33(5):509–515
Arora N, Dubey D, Sharma M, Patel A, Guleria A, Pruthi PA, Kumar D, Pruthi V, Poluri KM (2018) NMR-based metabolomic approach to elucidate the differential cellular responses during mitigation of arsenic(III,V) in a green microalga. ACS Omega 3(9):11847–11856
Ashok S, Senthil A, Sritharan N, Punitha S, Divya K, Ravikesavan R (2018) Yield potential of small millets under drought condition. Madras Agric J 105(7–9):370–372
Balasubramanian V, Adhya TP, Ladha JK, Hershey C, Neate P (2013) Enhancing eco-efficiency in the intensive cereal-based systems of the Indo-Gangetic Plains. Eco-efficiency: From vision to reality. CIAT, Cali, Colombia 2013:99–115
Baxte A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Berg G, Rybakova D, Fischer D, Cernava T, Verges MCC, Charles T, Chen X, Cocolin L, Eversole K, Corral GH, Kazou M (2020) Microbiome definition re-visited: old concepts and new challenges. Microbiome 8(1):1–22
Bhatt D, Negi M, Sharma P, Saxena SC, Dobriyal AK, Arora S (2011) Responses to drought induced oxidative stress in five finger millet varieties differing in their geographical distribution. Physiol Mol Biol Plants 17(4):347–353
Bouis HE (2000) Enrichment of food staples through plant breeding: a new strategy for fighting micronutrient malnutrition. Nutrition (Burbank, Los Angeles, Calif.) 16(7–8):701–704
Bukhat S, Imran A, Javaid S, Shahid M, Majeed A, Naqqash T (2020) Communication of plants with microbial world: exploring the regulatory networks for PGPR mediated defence signalling. Microbiol Res 238:126486
Camacho-Coronel X, Molina-Torres J, Heil M (2020) Sequestration of exogenous volatiles by plant cuticular waxes as a mechanism of passive associational resistance: a proof of concept. Front Plant Sci 11:121
Carrion VJ, Perez-Jaramillo J, Cordovez V, Tracanna V, De Hollander M, Ruiz-Buck D, Mendes LW, Van Ijcken WF, Gomez-Exposito R, Elsayed SS, Mohanraju P (2019) Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 366(6465):606–612
Chandrasekara A, Shahidi F (2010) Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J Agric Food Chem 58(11):6706–6714
Chen BJ, During HJ, Anten NP (2012) Detect thy neighbor: identity recognition at the root level in plants. Plant Sci 195:157–167
Che-Othman MH, Jacoby RP, Millar AH, Taylor NL (2020) Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol 225(3):1166–1180
D’alessandro M, Erb M, Ton J, Brandenburg A, Karlen D, Zopfi J, Turlings TC (2014) Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tri-trophic interactions. Plant Cell Environ 37(4):13–826
Deshpande SS, Mohapatra D, Tripathi MK, Sadvatha RH (2015) Kodo millet-nutritional value and utilization in Indian foods. J Grain Process Storage 2(2):16–23
Dwivedi SL, Upadhyaya HD, Senthilvel S, Hash CT, Fukunaga K, Diao X, Santra D, Baltensperge D, Prasad M (2012) Millets: genetic and genomic resources. In: Plant breeding reviews. Wiley-Blackwell, Hoboken, pp 247–375
Fernandez V, Ebert G (2005) Foliar iron fertilization: a critical review. J Plant Nutr 28(12):2113–2124
Foito A, Stewart D (2018) Metabolomics: a high-throughput screen for biochemical and bioactivity diversity in plants and crops. Curr Pharm Des 24(19):2043–2054
Fukushima A, Kusano M, Redestig H, Arita M, Saito K (2009) Integrated omics approaches in plant systems biology. Curr Opin Chem Biol 13(5–6):532–538
Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, Holub EB, Hammerschmidt R, Ausubel FM (1997) Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146(1):381–392
Gupta V, Jatav PK, Verma R, Kothari SL, Kachhwaha S (2017) Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ Sci Pollut Res 24(30):23915–23925
Hassan S, Mathesius U (2012) The role of flavonoids in root–rhizosphere signalling: opportunities and challenges for improving plant–microbe interactions. J Exp Bot 63(9):3429–3444
IIMR (2018) Annual Report 2017–2018. Hyderabad: Indian Institute of Millets Research
Imperiali N, Chiriboga X, Schlaeppi K, Fesselet M, Villacres D, Jaffuel G, Bender SF, Dennert F, Blanco-Perez R, Van der Heijden MG, Maurhofer M (2017) Combined field inoculations of Pseudomonas bacteria, arbuscular mycorrhizal fungi, and entomopathogenic nematodes and their effects on wheat performance. Front Plant Sci 8:1809
Iwuala E, Odjegba V, Umebese C, Sharma V, Alam A (2019) Physiological and gene expression studies of selected Zea mays L. and Pennisetum glaucum (L.) R. Br. genotypes to simulated drought stress condition. Vegetos 32(3):397–406
Jonak C, Okresz L, Bogre L, Hirt H (2002) Complexity, cross talk, and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5(5):415–424
Karban R (2008) Plant behaviour and communication. Ecol Lett 11(7):727–739
Khatoon H, Singh V (2016) Impact of water stress on physiological and biochemical parameters of finger millet (Eleusine coracana L.). Res Environ Life Sci 9(12):1474–1477
Kong HG, Song GC, Sim HJ, Ryu CM (2021) Achieving similar root microbiota composition in neighbouring plants through airborne signalling. ISME J 15(2):397–408
Kumar A, Sharma D, Tiwari A, Jaiswal JP, Singh NK, Sood S (2016) Genotyping-by-sequencing analysis for determining population structure of finger millet germplasm of diverse origins. Plant Genome 9(2):0058. https://doi.org/10.3835/plantgenome2015.07.0058
Lata C (2015) Advances in omics for enhancing abiotic stress tolerance in millets. Proc Indian Natl Sci Acad 81:397–417
Li LL, Zhao HH, Kong CH (2020) Loliolide, the most ubiquitous lactone, is involved in barnyard grass-induced rice allelopathy. J Exp Bot 71(4):1540–1550
Liang S, Yang G, Ma Y (2010) Chemical characteristics and fatty acid profile of foxtail millet bran oil. J Am Oil Chem Soc 87(1):63–67
Mhlongo MI, Piater LA, Madala NE, Labuschagne N, Dubery IA (2018) The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front Plant Sci 9:112
Mur LA, Prats E, Pierre S, Hall MA, Hebelstrup KH (2013) Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defence pathways. Front Plant Sci 4:215
Neelam Y, Kanchan C, Alka S, Alka G (2013) Evaluation of hypoglycemic properties of kodo millet-based food products in healthy subjects. J Pharm 3:14–20
Novoplansky A (2016) Future perception in plants. In: Anticipation across disciplines. Springer, Cham, pp 57–70
Odhong C, Wilkes A, Van Dijk S, Vorlaufer M, Ndonga S, Sing’ora B, Kenyanito L (2019) Financing large-scale mitigation by smallholder farmers: what roles for public climate finance? Front Sustain Food Syst 3:3
Osman HA, Ameen HH, Mohamed M, Elkelany US (2020) Efficacy of integrated microorganisms in controlling root-knot nematode Meloidogyne javanica infecting peanut plants under field conditions. Bull Natl Res Centre 44(1):1–10
Penaflor MFG, Bento JMS (2019) Red-rot infection in sugarcane attenuates the attractiveness of sugarcane borer-induced plant volatiles to parasitoid. Arthropod Plant Interact 13(1):117–125
Penuelas J, Asensio D, Tholl D, Wenke K, Rosenkranz M, Piechulla B, Schnitzler J (2014) Biogenic volatile emissions from the soil. Plant Cell Environ 37(8):1866–1891
Perez-Labrada F, Hernandez-Hernandez H, Lopez-Perez MC, Gonzalez-Morales S, Benavides-Mendoza A, Juarez-Maldonado A (2020) Nanoparticles in plants: morphophysiological, biochemical, and molecular responses. In: Plant life under changing environment. Academic Press, New York, pp 289–322
Qadir M, Bibi AB, Sadaqat HA, Awan FS (2019) Physio-biochemical responses and defining selection criteria for drought tolerance in Sorghum bicolor. Maydica 64(2):8
Quintana-Rodriguez E, Morales-Vargas AT, Molina-Torres J, Adame-Alvarez RM, Acosta-Gallegos JA, Heil M (2015) Plant volatiles cause direct, induced, and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J Ecol 103(1):250–260
Rahman MKU, Zhou X, Wu F (2019) The role of root exudates, CMNs, and VOCs in plant–plant interaction. J Plant Interact 14(1):630–636
Sabir P, Ashraf M, Hussain M, Jamil A (2009) Relationship of photosynthetic pigments and water relations with salt tolerance of proso millet (Panicum miliaceum L.) accessions. Pak J Bot 41(6):2957–2964
Saijo Y, Loo EPI (2020) Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol 225(1):87–104
Saikkonen K, Nissinen R, Helander M (2020) Toward comprehensive plant microbiome research. Front Ecol Evol 8:61
Saleh AS, Zhang Q, Chen J, Shen Q (2013) Millet grains: nutritional quality, processing, and potential health benefits. Compr Rev Food Sci Food Saf 12(3):281–295
Satish L, Rathinapriya P, Rency AS, Ceasar SA, Prathibha M, Pandian S, Rameshkumar R, Ramesh M (2016) Effect of salinity stress on finger millet {Eleusine coracana (L.) Gaertn}: histochemical and morphological analysis of coleoptile and coleorhizae. Flora Morphol Distrib Funct Ecol Plants 222:111–120
Saxena R, Vanga SK, Wang J, Orsat V, Raghavan V (2018) Millets for food security in the context of climate change: a review. Sustainability 10(7):2228
Saxena R, Kumar M, Jyoti A, Tomar RS (2019) Untapped potential of salicylic acid, jasmonic acid and PGPRs to develop abiotic stress resilience in crop plants. Curr Trends Biotechnol Pharm 13(4):376–390
Sharifi R, Lee SM, Ryu CM (2018) Microbe-induced plant volatiles. New Phytol 220(3):684–691
Sharma R, Kumar R, Satapathy SC, Al-Ansari N, Singh KK, Mahapatra RP, Agarwal AK, Le HV, Pham BT (2020) Analysis of water pollution using different physicochemical parameters: a study of Yamuna River. Front Environ Sci 8:581591
Shulaev V, Cortes D, Miller G, Mittler R (2008) Metabolomics for plant stress response. Physiol Plant 132(2):199–208
Singh KP, Mishra HN, Saha S (2010) Moisture-dependent properties of barnyard millet grain and kernel. J Food Eng 96(4):598–606
Singh RK, Muthamilarasan M, Prasad M (2021) Biotechnological approaches to dissect climate-resilient traits in millets and their application in crop improvement. J Biotechnol 327:64–73
Sobiczewski P, Lakimova ET, Mikicinski A, Wegrzynowicz-Lesiak E, Dyki B (2017) Necrotrophic behaviour of Erwinia amylovora in apple and tobacco leaf tissue. Plant Pathol 66(5):842–855
Sood S, Khulbe RK, Gupta AK, Agrawal PK, Upadhyaya HD, Bhatt JC (2015) Barnyard millet—a potential food and feed crop of future. Plant Breed 134(2):135–147
Stamp N (2003) Out of the quagmire of plant defence hypotheses. Q Rev Biol 78(1):23–55
Sun ZC, Yu QL, Lin H (2015) The environmental prospects of cultured meat in China. J Integr Agric 14(2):234–240
Sung J, Lee S, Lee Y, Ha S, Song B, Kim T, Waters BM, Krishnan HB (2015) Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus, or potassium-deficient condition. Plant Sci 241:55–64
Tadele Z (2016) Drought adaptation in millets. Intech, London, pp 639–662
Thathola A, Srivastava S, Singh G (2011) Effect of foxtail millet (Setaria italica) supplementation on serum glucose, serum lipids and glycosylated hemoglobin in type 2 diabetics. Diabetologia Croatica 40(1):23–29
Ugare R, Chimmad B, Naik R, Bharati P, Itagi S (2014) Glycemic index and significance of barnyard millet (Echinochloa frumentacae) in type II diabetics. J Food Sci Technol 51(2):392–395
Vahabi K, Reichelt M, Scholz SS, Furch AC, Matsuo M, Johnson JM, Sherameti I, Gershenzon J, Oelmuller R (2018) Alternaria brassicae induces systemic jasmonate responses in Arabidopsis which travel to neighbouring plants via a Piriformospora indica hyphal network and activate abscisic acid responses. Front Plant Sci 9:626
Van Dam NM, Bouwmeester HJ (2016) Metabolomics in the rhizosphere: tapping into belowground chemical communication. Trends Plant Sci 21(3):256–265
Vickers NJ (2017) Animal communication: when I’m calling you, will you answer too. Curr Biol 27(14):R713–R715
Wang L, Wang XY, Wen QF, Wu B (2005) Research and utilization of proso millet germplasm resource in China. J Plant Genet Resour 6(4):474–477
Wenig M, Ghirardo A, Sales JH, Pabst ES, Breitenbach HH, Antritter F, Weber B, Lange B, Lenk M, Cameron RK, Schnitzler JP (2019) Systemic acquired resistance networks amplify airborne defence cues. Nat Commun 10(1):1–14
Wood NT, Allan AC, Haley A, Viry-Moussaid M, Trewavas AJ (2000) The characterization of differential calcium signalling in tobacco guard cells. Plant J 24(3):335–344
Zahar Haichar F, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–68
Zhang N, Wang D, Liu Y, Li S, Shen Q, Zhang R (2014) Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 374(1):689–700
Zhang H, Li Y, Zhu JK (2018) Developing naturally stress-resistant crops for a sustainable agriculture. Nat Plants 4:989–996
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mazumdar, S. et al. (2023). Understanding of Belowground Biochemical Communication in Millets Through Metabolomics. In: Pudake, R.N., Kumari, M., Sapkal, D.R., Sharma, A.K. (eds) Millet Rhizosphere . Rhizosphere Biology. Springer, Singapore. https://doi.org/10.1007/978-981-99-2166-9_13
Download citation
DOI: https://doi.org/10.1007/978-981-99-2166-9_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2165-2
Online ISBN: 978-981-99-2166-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)