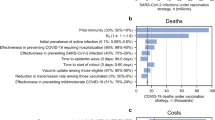
Abstract
The scientific community has achieved a remarkable feat by developing COVID-19 vaccines in a record duration of 12 months. The fastest vaccine developed and deployed previously was within a time-frame of four years, to prevent mumps in the 1960s. The speedy approach to prevent SARS-CoV-2 has changed the future of vaccine science with several vaccines showing excellent results in large trials. The COVID-19 vaccination strategy is of crucial importance for controlling the pandemic. As the vaccination mandate was faster than information dissemination, and even faster than the clinical trial results in some regions, numerous challenges emerged during the implementation of the vaccination strategy. The author discusses the objectives of the vaccination drive that include: reduction of overall COVID-19 severity and mortality; re-opening of society; disease elimination; reduction of pressure on the healthcare system; and equitable distribution of vaccines across all regions of the globe. While reflecting on these objectives, the author discusses the importance of transparency in vaccination surveillance data and the way forward.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Background
As of March 28, 2022, 6.12 million confirmed deaths and 484 million confirmed cases of infection with SARS-CoV-2, the virus causing COVID-19, were reported globally [1]. When the World Health Organization (WHO) declared COVID-19 as a pandemic on March 11, 2020, the entire world went into a panic trying to figure out possible strategies to adopt in order to control the spread of COVID-19. During the last 20 months, we have come a long way. State administrations have been busy strategizing pre-emptive measures to control the rapidly spreading pandemic including lockdowns and strengthening the healthcare system. International organizations have framed directive steps for countries to follow. At the same time, scientists all over the world have been busy developing vaccines and therapeutics. Scientists have spent the past year developing vaccines and ensuring their safety and effectiveness. On December 3, 2020, the first ever COVID-19 vaccine, AstraZeneca, was approved by the United Kingdom. And on December 31, 2020, WHO approved the first vaccine, the Pfizer vaccine. To date, WHO has approved ten vaccines. And many are in the pipeline [2].
Although scientists have successfully developed vaccines, making them available to people has been an immense challenge. Vaccine deployment required resources, large manufacturing plants, and efficient supply chains to reach the remotest areas of the world and to overcome vaccine hesitancy. Vaccine development and an effective vaccine deployment strategy, as part of the pandemic response, are critical for achieving comprehensive vaccination coverage across countries.
There are no shortcuts to building vaccine development capacities. The differences in vaccination rates in high-income and low-income countries underscore that. However, low-income countries must adopt advanced technologies and combine them with a human touch to reasonably respond to this and future crises. “Charity is good, but we cannot rely on charity alone,” says Peter Singer, adviser to the Director General of the World Health Organization (WHO) [4]. Wealthy countries led the frontline and secured enough vaccines. However, the inequitable and inefficient skewed production and distribution of COVID vaccines in favor of rich countries left low- and middle-income countries dangerously unprotected [5]. Governments and companies that have developed highly effective vaccines must share patented knowledge and technology so that manufacturers in poor countries can produce vaccines for their populations. Existing vaccines like Pfizer et al. deployed in over 100 countries, if patented to be manufactured in the global South, could tame the pandemic and build vaccine confidence [4]. Health advocacy organizations have pointed to the deployment of Sputnik V vaccine as a model of pandemic diplomacy as Russia broadly licensed the vaccine outside its borders to 34 drug companies.
“High and upper-middle-income countries represent 53% of the world's population but have received 83% of the world's vaccines. In contrast, low and lower-middle-income countries account for 47% of the population but have received just 17% of the world's vaccines”, says the WHO Director General, Tedros Adhanom Ghebreyesus [7]. Table 3 shows the ranking of high to low-income countries based on their population size. To understand the trend of COVID-19 vaccination, data from 15 countries with the highest populations was analyzed. Of the 15 countries, only four high and upper-middle-income countries (China, the United States, Brazil, and Japan) had fully vaccinated 50% or more of their populations in 2021. On the other hand, countries like the Philippines, India, and Bangladesh with the highest population densities 368, 464, and 1,265 per square kilometer of land area, respectively were able to vaccinate only 25% of their populations [8, 9]. This gap increased with the emergence of COVID-19 variants. One such situation was seen in India when the country reported 3,876 deaths in every 24 h in April 2021 with the B.1.617 variant, designated as a ‘Variant of Concern’.Footnote 1 The country expressed its concern about the short supply of vaccines despite producing 70 million vaccine doses per month. India demanded that the time-table of supplies be made public to ensure transparency so that people can patiently wait for their turn [7]. The B.1.617 variant of SARS-CoV-2 increased rapidly with WHO reporting its spread in over 44 countries by May 2021. This variant catalyzed the third wave in the United Kingdom (UK) in June 2021 [10, 11]. This situation that the world has experienced demands that there should be equitable vaccine distribution.
Since vaccines were developed and deployed, more than 3.65 billion (47%) people worldwide received one dose of the COVID-19 vaccine. But only 36% of the people were fully vaccinated. Countries with fewer people are leading the charts of fully vaccinated populations. Sixteen countries successfully vaccinated 75% of their populations with both doses [9]. When the vaccination rate was analyzed, the picture began to look brighter for high-income countries like the United States and Europe as new COVID cases started to decline in these countries. However, in the Global South, COVID seemed to have become a disease of low-income countries, especially African countries [5]. While 6.2 billion vaccine doses were administered worldwide by October 2021, only 20% of the population in low and lower-middle-income countries received one dose of the vaccine. When compared to 50% of adults fully vaccinated in high-income countries, only 2% of adults in Africa were fully vaccinated [12, 13]. This vaccinated population includes the age group of 18 years and above.
Although there is a much lower risk of severe COVID-19 in children, it is important that they are vaccinated. The Pfizer vaccine, approved by the Food and Drug Administration (FDA) in the United States for children 12 years or older, will soon be approved for children aged five years and above. Clinical trials, already underway, have shown that it is 90.7% effective in preventing COVID-19 in children. The Moderna and Johnson & Johnson vaccines are authorized for the age group 18 and above. There is, however, concern regarding adverse events post-vaccination. Studies are being conducted in 5–11-year-old children to test the vaccines before initiating vaccination programs [14]. The UK started vaccinating the population of 12 to 15 years with the recommendation of the Joint Committee on Vaccination and Immunization (JCVI) and strategized to initiate a school-based immunization program with parental, guardian, or carer consent. This strategy is similar to a previously tested way of administering vaccines to children to prevent HPV and diphtheria, pertussis, and tetanus (DPT) [15]. Countries across Europe, the Asia Pacific, the Middle East, the Americas, and South Africa have started to design strategies to vaccinate children above 12 years of age [16]. A survey conducted by the Angus Reid Institute in Canada showed that 50% of parents plan to get their children vaccinated when vaccines are approved and made available [17].
As high-income countries started to plan vaccinating children 5–11 years of age, international organizations, governments, and international NGOs came forward to support low-income countries. Inequitable distribution and its impact on low-income countries were evident. The WHO urged companies and countries to prioritize the supply of COVID-19 vaccines through the COVID-19 Vaccine Global Access Facility, COVAXFootnote 2 [16]. Working in partnership with developed and developing countries, COVAX is the only global initiative that is working with governments and manufacturers to ensure that COVID-19 vaccines are available to low-income countries [18]. COVAX is pooling buying power from participating economies to provide access to COVID-19 vaccines. But pooling resources was challenging as COVAX needed unprecedented cooperation from governments, researchers, manufacturers, and multilateral partners. Over 1.2 billion doses were pledged to be donated by COVAX and G7 as bilateral supply donations by mid-2022. However, till September 2021 only 12% were delivered [19]. The commitment of highly vaccinated countries to vaccinate the rest of the world has yet to be realized.
“In the scramble for a vaccine, countries can act alone—creating a few winners and many losers—or they can come together to participate in COVAX, an initiative which is built on enlightened self-interest but also equity, leaving no country behind,” said Richard Hatchett, Chief Executive Officer of the Coalition for Epidemic Preparedness Innovations (CEPI).
As the crisis exposed significant fragilities in the world’s capacity to prevent and respond to health emergencies, countries took informed decisions with the help of extraordinary scientific advancement. To ensure that we gain from this momentum of innovation, there is a need to analyze and capture every step of this scientific journey to build capacities, prevent and respond to health emergencies, and strengthen the health system. The author of this chapter aims to capture the progress that has been made by countries by adopting various strategies. The author discusses COVID vaccines developed and the challenges faced by countries during vaccine deployment. An attempt is also made to analyze vaccination rates and vaccine equity based on the country's population density and income level.
Vaccination Rates of Countries
As countries prepared to implement COVID-19 vaccination programs in November 2020, a three-step framework was developed by the Strategic Advisory Group of Experts (SAGE). This framework provides a prioritized roadmap for vaccination programs. It makes recommendations for allocating vaccines for countries and prioritizing various population groups. The framework also addresses ethical issues. To vaccinate the population based on priority, several stages were defined. In Phase 1, 1% to 20% of the country's population should be vaccinated. As the vaccine supply increases, vaccines can be made available to 20% and more of the population [21]. The framework defines the COVID-19 vaccine as a global ‘public good’Footnote 3 and requires that the vaccine should contribute to the equitable protection and promotion of human wellbeing among all the people of the world [22].
Since SAGE published its guidelines almost 12 months ago, most countries have made some progress. Taking the SAGE prioritization framework as a beginner’s guide, countries developed their vaccination strategies and vaccinated their populations based on priority groups. Vaccination programs were rolled out by most of the countries in early 2021. Four Variants of Concern emerged resulting in a surge of cases. This led to a rethinking of herd immunity and short-and long-term immunity. As we are still learning the science and the math of population immunity, it is difficult to say how many people need to be vaccinated against COVID-19 to attain herd immunity [23]. Some experts estimate that herd immunity will be reached when 70% to 90% of the population is vaccinated [24].
Box 1 Principles
1. Human wellbeing: Protect and promote human wellbeing including health, social and economic security, human rights, civil liberties, and child development
2. Equal respect: Recognize and treat all human beings as having equal moral status and their interests as deserving of equal moral consideration
3. Global equity: Ensure equity in vaccine access and benefits globally among people living in all countries, particularly those living in low and middle-income countries
4. National equity: Ensure equity in vaccine access and benefit within countries for groups experiencing a greater burden of COVID-19 infection
5. Reciprocity: Honor obligations of reciprocity for those individuals and groups within countries who bear significant additional risk and burden of COVID-19 infection
6. Legitimacy: Make global decisions about vaccine allocation and national decisions about vaccine prioritization through transparent processes that are based on shared values, best available scientific evidence, and appropriate representation of affected parties
Source World Health Organization SAGE Values Framework for Allocation and Prioritization of COVID-19 vaccination.
Data recorded worldwide shows that more than 16 countries were able to vaccinate 75% or more of their populations by October 2021. Figure 1 shows the trend of fully vaccinated populations from West to East irrespective of population size. It shows that the northern and western countries are rapidly immunizing their populations against the COVID virus [25]. By July 2021, four billion vaccine doses were administered of which less than one percent were administered in low-income countries and eight out of ten doses were used to vaccinate people in wealthy countries, ultimately, putting the low-income countries in a vulnerable position [26].
People vaccinated fully and partially against COVID-19 by country
Source Official data collated by World in Data, October 25, 2021
Note Alternative definitions of a full vaccination, e.g., having been infected with SARS-CoV-2 and having 1 dose of a 2-dose protocol, are ignored to maximize comparability between countries
Countries with highly vaccinated populations, high density, and large populations were grouped together in Table 3 to analyze the success of vaccination strategies and to understand the effectiveness of the national COVID-19 response. Among these countries, only China, Brazil, and Japan were able to fully vaccinate (75%) of their populations. These countries are stepping towards population immunity and will develop herd immunity based on the claims that some of the researchers have made. Countries with large populations were specifically picked to understand the path of vaccination and how they managed to vaccinate large populations. The population density of countries was assessed to understand how it can impact vaccination strategies and how far are we in our efforts to end this pandemic.
In earlier research conducted on smallpox comparing population densities in the Indian subcontinent and Africa showed that the impact of population density was significantly linked to the impact of immunization programs. The analysis showed that the percentage of the population with pockmarksFootnote 4 in densely populated areas was higher when compared to those that were not densely populated [27].
Viruses usually spread at the local level. This statement is well supported by how measles was declared to have been eliminated from the U.S. in 2000, but reappeared in recent years because vaccination rates were low in some pockets of the country which was where measles outbreaks occurred [28].
To analyze progress made towards achieving population immunity, countries with large population sizes were placed along with countries that were performing exceptionally well in their COVID vaccination programs. The countries selected are presented in Table 1 along with their population size ranking. Figure 3 shows COVID-19 vaccine doses administered to the population of the country as on October 24, 2021. It shows that India and China have already crossed the mark of administering one billion vaccine doses. This was achieved in a period of nine months in India. This was shorter for India than for all other countries.
Since April 2020, high-income countries have been asserting that ‘no one is safe until we are all safe’. They have been ‘promising to make vaccination a truly unique, global public good’. And yet, these countries have consistently undermined proposals to achieve equitable production, supply, and distribution of vaccines. And while commitments of making the vaccines are touted by these countries, only a fraction of the doses needed have, in fact, been shared.
Challenges
Patents on COVID-19 Vaccines
During the early days of vaccine development, patents on COVID-19 vaccines retarded vaccine development in low-income countries. There was a lot of debate about whether or not rich countries should waive their intellectual property rights on vaccines. Various international forums established panels to discuss this issue involving European and US economists. One such forum called the Initiatives on Global Markets (IGM) undertook a survey in which 87% of the panelists agreed that rather than waiving their intellectual property protection, rich countries should pay for 12 billion vaccine doses to pharmaceutical companies to manufacture and distribute vaccines to developing countries. The majority of the arguments were in favor of rich countries. It was found that many of the respondents were worried about the effects on future innovations of waiving patents today. In the same poll, 89% of the panelists agreed that even the benefits of providing vaccine for free outweigh the costs that the rich countries would have to bear if the pandemic does not end sooner [29].
“The problem with waiving patent protection is that the manufacturing components may not be available to low-income countries” says Carol Propper, Imperial College [29].
Box 2 What does waiving of intellectual property rights on the COVID-19 vaccine mean?
A patent is a powerful intellectual property right that grants an exclusive monopoly to the inventor for a limited, pre-specified time. It is granted by the government and provides an enforceable legal right against copying of the invention. There are product and process patents
Product patent: A product patent ensures a right to the final product during a specified time-period even if the product is made using a different technology
Process patent: A process patent prevents any person, other than the patent holder, to manufacture the product by modifying the manufacturing process
With Emergency Use Authorization (EUA), the waiving of intellectual property rights (IPR) means that vaccines—such as those developed by Pfizer, Moderna, AstraZeneca, Novavax, Johnson & Johnson, and Bharat Biotech—open up space for the production of vaccines on a larger scale in middle-income countries through licensing and technology transfer [30].
“Vaccinating the world has a strong positive externality: it reduces the scope for future virus mutations. Gains exceed costs for rich countries” by Kjetil Storesletten, Professor Department of Economics, University of Oslo [29].
Falsified Vaccines
Falsified vaccines are fake vaccines that are designed to mimic real vaccines. These vaccines do not comply with intellectual property rights (IPR) and the infringe trademark law. The WHO published an alert on its website related to the falsified COVID-19 Covishield vaccine identified in Africa and Asia [31]. This is a serious issue for countries receiving help from COVAX and bilateral tie-ups. It is only now that high-income countries have started providing promised doses to low- and low-middle-income countries. A lot of resources, from procuring to dissemination and administration, are consumed to make vaccines available. Falsified vaccines are delivered at vaccine sites because of corruption at the regional level. This calls into question the credibility of vaccine donors and leads to vaccine hesitancy.
Waning Immunity and Need for Booster Shots
Studies suggests, there is a steady decline of antibody level among vaccinated individuals overtime. This means that there is waning immunity. The healthcare records of countries show decreased protection than was initially. This has given rise to the much-needed booster shots which are particularly for the elderly and vulnerable groups. However, all these efforts could be wasted as SARS-CoV-2 becomes endemic with seasonal outbreaks. With insufficient vaccination coverage in developing countries depends on developed countries providing adequate COVID vaccine doses [32].
Vaccine Administration
Half the world’s population has received at least one dose of the COVID-19 vaccine. But figures vary widely among countries. For example, in July 2021, four billion vaccine doses were administered, of which less than one percent went to low-income countries and eight out of ten went to people in wealthy countries. Vaccine inequity and surging COVID variants have placed low-income countries in a vulnerable situation. Increasing availability of safe and effective vaccines gives hope of bringing the pandemic under control with very low mortality. However, the probable trajectory for SARS-CoV-2 is to become endemic with seasonal outbreaks. Because of waning immunity, insufficient vaccination coverage globally, and/or the emergence of new viral variants that current vaccines do not prevent, additional epidemic waves are likely, particularly in countries with low vaccination coverage [32]. The Global Health Summit report includes 10 critically urgent recommendations for global health threat prevention, preparedness, and response:
-
1.
End the acute stage of the pandemic and leave no one behind: Ensure equitable access to medical tools to fight COVID-19.
-
2.
Invest in scientific research and development before, during, and between health crises.
-
3.
Actively and genuinely involve research groups from low- and middle-income countries.
-
4.
Strengthen integrated disease surveillance, data collection, analysis, and sharing at all levels.
-
5.
Strengthen and protect science advice.
-
6.
Be ready for the next health crisis: invest in strengthening health systems and the workforce for preparedness and response.
-
7.
Strengthen regional manufacturing capacities and hubs.
-
8.
Empower people and earn their trust.
-
9.
Collaborate and coordinate at all levels; strengthen relevant governance structures and leadership, and ensure adequate financing.
-
10.
Scale-up production and promote equitable distribution of vaccine doses worldwide [32, 33].
Scaling Up Vaccine Production and Distribution
More than 200 clinical trials and close to 300 partnerships and collaborations among manufacturers have resulted in increasing the production of vaccines from zero to 7.5 billion COVID-19 vaccine doses in just ten months. The International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), headquartered in Geneva represents research-based pharmaceutical companies, has official relations with the United Nations and helps to develop and provide medicines and vaccines worldwide. IFPMA has described five steps to help scale-up vaccine production and maintain vaccine equity [34, 35].
-
1.
Stepping up production and dose sharing
-
2.
Maximizing COVID-19 vaccine output without compromising its safety and quality
-
3.
Eliminating trade barriers
-
4.
Supporting countries, especially LMICs and LICs, to deploy available doses of vaccines
-
5.
Developing new and next generation COVID-19 vaccines [36].
With the current manufacturing scale, IFPMA believes that 12 billion vaccine doses will be produced by the end of 2021 and 24 billion by 2022 provided there are no bottlenecks. However, these will be insufficient if low and lower-middle-income countries are not provided with enough support [34]. Airfinity indicates that there will be enough vaccines with G7 countries to vaccinate teenagers and adults and give booster doses to at risk populations in 2021. In addition to this, 1.2 billion doses will be available for redistribution [37]. However, timing for the scale-up and scale-out of vaccine manufacturing remains uncertain if there are bottlenecks in the supply of raw materials.
Surplus Doses
Airfinity’s analysis report on vaccine stocks in Western countries that include the U.S., UK, European Union, Canada, and Japan predicted that 500 million vaccine doses were available for redistribution in September 2021. Out of that stock, 360 million doses were not earmarked for donation. This prediction was made based on the available supply of vaccines within countries and the amount of vaccines that were available to be sent elsewhere [38]. The report showed that high-income countries are stockpiling plenty of vaccines which could otherwise be supplied globally. As vaccines have a shelf life, this could result in wastage of stockpiled vaccines without meeting the global demand.
Extra Doses
Airfinity collected data based on three main inputs for each manufacturing facility globally—the company’s stated production, the real observed production, and assumption of time needed for scaling up—to determine vaccine production and delivery to each site. In May 2021, Airfinity predicted that production could exceed 10 billion vaccine doses to reach the manufacturing phase by the end of 2021 [39]. This prediction was further confirmed by Airfinity in September when it predicted that vaccine manufacturers were producing 1.5 billion doses per month and with the expected scaling, this would reach 11.3 billion vaccine doses required to vaccinate the world’s population [19]. Airfinity also predicted that even after providing booster shots to adults, over 1.2 billion doses would be available for donation by the G7 alone in 2021. It suggested that 258,765,005 vaccine doses could be distributed per month given currently pledged donations and available stocks. It also predicted that the G7 countries would waste 241 million doses by the end of 2021. Therefore, these doses are distributed immediately [40]. This G7 available stock could allow LIC/LMIC to vaccinate 70% of their populations [19, 41]. Over 1.2 billion doses were pledged to be donated by COVAX and bilateral supply donations from G7 by mid-2022, of which till September 2021, only 12% were delivered.
Vaccine Wastage
A study conducted by Airfinity showed that vaccines were not being utilized properly. Monopolized vaccine delivery contracts resulted in the wastage of 100 million unused vaccine doses lying with western countries. Allowing for the wastage of 100 million plus doses is equivalent to putting hundreds of thousands of unvaccinated people in LICs to unnecessary suffering and death [13].
Vaccine Inequity
Global vaccine equity, widespread acceptance, and efficient deployment are moral imperatives for pandemic control. No country is safe unless all countries are safe. Thus, redistribution of vaccines, funding international platforms, and increasing availability and manufacturing capacity of vaccines is crucial for bringing the pandemic under control and preventing future epidemics from escalating into global emergencies. Inequitable access not only prevents mortality and suffering, but also hampers critical control efforts globally. Such geographies serve as breeding grounds for the emergence of viral mutants which could lead to ‘immune escape variants’ resistant to current vaccines and antibody based therapeutics. There is a continuing need for non-pharmaceutical interventions to prevent outbreaks until sufficiently high immunization levels are reached globally and it becomes clear how effective vaccines are in preventing viral transmission. The future of the pandemic will also depend on how effectively and rapidly countries can control small outbreaks [32].
Role of International Bodies
The role of international organizations working globally is important for controlling the COVID-19 pandemic as they galvanize action, maintain global mitigation efforts, build resources, prevent continual resurgence, and combat future global health crises.
Global Alliance for Vaccines and Immunizations (GAVI)
Established in 2000, the Global Alliance for Vaccines and Immunizations (GAVI) is a public–private partnership that has helped to vaccinate half the world’s children against some of the world’s deadliest diseases. GAVI has helped to immunize over 888 million children and has prevented 15 million deaths. And over the years, GAVI has helped to halve child mortality in 73 low-income countries. GAVI played a role in strengthening global health systems as well as in providing funds for Ebola, cholera, meningitis, and yellow fever vaccines. Employing innovative financing and the latest technology, GAVI has saved many lives and has prevented several outbreaks. GAVI is funded by governments, corporations, foundations, and private individuals. As a co-founder of COVAX, GAVI is focused on procurement and delivery for COVAX with its Alliance partners United Nations Children’s Emergency Fund (UNICEF) and WHO along with national governments [42].
COVID-19 Vaccine Global Access Facility (COVAX)
COVID-19 Vaccine Global Access Facility (COVAX), an initiative of 178 countries, is one of the three pillars under the Access to COVID-19 Tools (ACT) Accelerator. It pools economic resources of its member countries to enable equitable access to vaccines. The two main objectives of this initiative are: (1) to enable vaccine developers to make high-risk investments for the development of vaccines; and (2) to subsidize vaccine costs for middle- and low-income countries.
COVAX coordinated by GAVI, the Vaccine Alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and the WHO acts as a platform for a wide range of research, development, manufacturing, and price negotiations of COVID-19 vaccine candidates. With the initial aim of making available two billion doses by the end of 2021 for high-risk, vulnerable frontline workers, COVAX created the most diverse portfolio of vaccines with nine candidates already in development and a further nine under evaluation. COVAX promises to provide to 170 countries, with sufficient guaranteed doses irrespective of funding or self-financing [43]. In addition, 78 high-income economies have confirmed their interest in supporting the COVAX Facility.
COVAX has established a COVAX Manufacturing Task Force that coordinates with the Coalition for Epidemic Preparedness Innovations (CEPI), GAVI, WHO, and UNICEF and partners with the Bill & Melinda Gates Foundation, International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), Developing Countries Vaccine Manufacturing Network (DCVMN), and Biology Investigative Opportunities (BIO). The COVAX Task Force aims to advance the work and partnerships of COVAX with the longer-term aim to help strengthen regional health capacities to respond to such crises in the future. The COVAX Manufacturing Task Force has specific objectives categorized as short-, medium-, and long-term [44]. Its short-term objective is to establish global trade, promoting cross-border trade, promote voluntary partnerships, and support vaccine development. Its mid- to long-term objective is to upgrade vaccine manufacturing and raise funds for LMICs.
Vaccines need to be allocated simultaneously to all participating countries proportional to their total population size with a five percent buffer kept aside to build a stockpile. No country will receive more than 20% of the vaccine for its population until all countries receive the same amount of vaccine [43]. By October 2021, COVAX shipped over 406 million COVID-19 vaccine doses to 144 participant countries [45].
GAVI-COVAX-AMC
To arrange funds for 92 middle-and lower-income countries that cannot fully afford to pay for COVID-19 vaccines, a separate arm of the COVAX facility called the GAVI-COVAX-AMC arm, focuses primarily on providing equal access of COVID-19 vaccines to middle- and low-income countries at the same time as to high-income countries. GAVI-COVAX-AMC receives its funds from the private sector, philanthropies, and Official Development Assistance (ODA) [43].
Funding is now coming from various sources like the recent deal with six Gulf countries to support COVAX and GAVI-COVAX-AMC. Over 9.8 billion USD in funding were pledged by donors to GAVI-COVAX-AMC and almost 600 million doses have been received to date reserving five percent of the funds to be used as the ‘humanitarian buffer’—a key component of the COVAX buffer—for high-risk populations in humanitarian settings [42]. The UN Security Council also passed a resolution in 2021 making it the obligation of countries to ensure access to COVID-19 vaccines for populations in humanitarian settings [46]. Hoping to receive continuous support, COVAX has started delivering COVID vaccine stocks to countries.
Coalition for Epidemic Preparedness Innovations (CEPI)
The Coalition for Epidemic Preparedness Innovations (CEPI) was launched in 2017 as part of a 3.5 billion dollars action plan that forged partnerships with over 30 vaccine developers, academic institutions, and manufacturers. Member countries include European nations and 30 other countries and philanthropic organizations—the Bill and Melinda Gates Foundation and the Wellcome Trust. In addition, CEPI, alongside GAVI and the WHO launched COVAX to enable equitable access of COVID-19 vaccines across nations.
Scientists believe that the coronavirus family is a significant recurrent pandemic threat. Two classes of coronavirus, SARS and MERS are deadly with fatality rates of 10%-35% [47]. To reduce the threat of future outbreaks, CEPI has set the following objectives:
-
1.
Compress vaccine development time-lines to 100 days: Scientists developed COVID-19 vaccines in a record time of 300 days. CEPI aims to reduce this time-line of vaccine development further to 100 days
-
2.
CEPI aims to develop a universal vaccine against coronavirus: A broadly protective vaccine to eliminate the risk of the existing coronavirus and future risk of other possible coronaviruses.
-
3.
Develop a library against other threats: Previous work on MERS enabled us to spring on to COVID-19 vaccine development. A library will be helpful in addressing newly emerging threats [47].
World Health Organization (WHO)
The World Health Organization (WHO) is working with partners to develop, manufacture, and deploy safe and effective vaccines. WHO has evaluated six vaccines against COVID-19 that met the criteria for safety and efficacy WHO is assisting COVAX, the Country Readiness and Delivery (CRD) workstream, the Pan American Health Organization (PAHO), and the United Nations Children's Fund (UNICEF) to provide safe and effective vaccines worldwide, especially in low-middle-income countries [48]. However, the goal to vaccinate at least 10% of the population of every low-income country remains unrealized. This is mainly due to the monopolized delivery contracts of western countries [13].
To ensure transparency, WHO developed regional level dashboards—the PAHO COVID-19 vaccines delivery dashboard and the AFRO COVID-19 vaccine dashboard. These dashboards helped to enhance the transparency and efficacy of vaccines delivered to Africa and the Latin American regions. In addition, in creating regional dashboards, WHO is collecting and collating data from various platforms and is publishing reports related to vaccine deployment. These reports are published weekly on issues like the disease profile of post-vaccination infection, surveillance of adverse events, vaccine usage, and falsified vaccine stocks. As an international body, WHO has a significant role to play in ensuring transparency and building vaccine confidence by sharing vaccine-related information. In addition, it can put pressure on developed economies to support middle- and low-middle-income countries to ensure equitable distribution of vaccines. Despite committing to donate vaccines, high-income countries have shared only a fraction of the doses needed by developing countries [31].
Pan American Health Organization (PAHO)
The Pan American Health Organization (PAHO) is a specialized international health agency for the Americas [49]. Along with its 51 member countries, PAHO engages in technical cooperation to fight communicable and non-communicable diseases. It is committed to strengthening the healthcare system and ensuring that all people can access healthcare during emergencies and disasters. PAHO aligned with WHO and its partners (The Revolving Fund and others) to pave the way for implementation of the COVAX Facility. PAHO is currently monitoring international logistics for 36 countries in the Americas region and, as part of the preparations, has reviewed the countries’ national vaccination and deployment strategies offering feedback and technical assistance. PAHO works with local health authorities to ensure that people receive the required doses of the vaccine. The first COVAX doses arrived in Latin America in the first week of March 2021 [50]. Figure 6 shows the data of the COVAX Facility, doses delivered, doses in transit, and total doses administered. The PAHO platform demonstrates how PAHO and COVAX work to achieve equitable distribution of COVID-19 vaccines to middle-and low-income countries.
G7 Countries
G7, an inter-governmental political forum that includes Canada, France, Germany, Italy, Japan, the United Kingdom, and the United States, is the world’s largest and wealthiest liberal democracy [51]. In March 2021, G7 published a statement to enhance its efforts to strengthen health systems within countries and globally. It mentioned supporting WHO in its global mandate to lead on disease outbreaks and emergencies [52]. The G7 countries achieved a vaccination rate of 60% and above of fully vaccinated people against COVID-19 in 11 months. But efforts to help the rest of the world fell far behind [25]. It was not until June 2021, after the G7 leader’s summit, that it came forward to support the rest of the world with a billion vaccine doses having shared 200 million doses by the end of 2021. The G7 countries are providing vaccine doses. But the need is much higher in real-time to save 60,000 lives as analyzed by the analytics company Airfinity [53, 54].
United Nations Children's Fund (UNICEF)
The United Nations Children's Fund (UNICEF) works in over 190 countries and territories for child protection, survival, education, social policies, gender issues, emergencies, and supplies and logistics. As the most significant single vaccine buyer globally, UNICEF harnessed its decades of expertise to deliver COVID-19 vaccines. In collaboration with PAHO, it procured and supplied authorized vaccines to 92 low and lower-middle-income countries [55]. UNICEF aims to deliver two billion doses of COVID-19 vaccines globally by the end of 2021 [56].
UNICEF and GAVI have been installing solar refrigerators across countries since 2018. During the COVID-19 pandemic, an early mapping was carried out of the necessary equipment that was missing at various locations while COVID-19 vaccines were being developed. When the impact of COVID-19 became evident in 2020, UNICEF, along with regional governments and partners, began to take stock of the number of vaccine doses required to impact the pandemic. As countries introduced travel restrictions, UNICEF was on the ground and was working round the clock with governments, COVAX Facility, and partners to transport COVID-19 vaccines to West and Central Africa and other difficult to reach locations [55].
Developing Countries Vaccine Manufacturing Network (DCVMN)
The Developing Countries Vaccine Manufacturing Network (DCVMN), a network of 19 countries, provides a platform for organizations to come together regularly to share technical information, best practices, and prospects related to COVID-19. Its member countries are engaged in deploying platforms for research, development, manufacturing, and scaling up the COVID-19 vaccine as gene sequencing is becoming available. DCVMN members scaled up production from zero to millions of doses in a record span of 10–12 months. They also licensed pharmaceutical companies like AstraZeneca and Johnson & Johnson to ensure the availability and affordability of vaccines to millions of people in LICs and LMICs.
The Serum Institute of India (SII) was the first organization that supported COVAX to supply vaccines to Africa. It’s most remarkable achievement, as a member of the DCVMN, was that it produced and supplied around 200 million doses globally to cater to the needs of various countries worldwide [34]. When the second wave hit India, there was a pushback in the delivery of vaccines in the national interest. Nevertheless, the delivery of vaccines was resumed and will hopefully continue until the pandemic ends. There is a need to enhance global collaboration to ensure equitable vaccine access. Hence, a more robust and effective global preparedness and response architecture is required. This means that a global research and manufacturing network needs to be in place.
The Bill and Melinda Gates Foundation (BMG)
The Bill & Melinda Gates Foundation’s (BMG’s) footprint is all over the COVID-19 response. The Foundation provided 1.8 billion USD in funding. It was an early investor in messenger RNA vaccines and other technologies instrumental in the fight against COVID-19. It emphasized three core priorities:
-
1.
Containing the global COVID-19 crisis
-
2.
Rebuilding and accelerating economic recovery
-
3.
Better preparing the global system for future pandemics
The BMG supported lower-income countries by strengthening their capacities, coordination, and funding for the development of new tests, treatments, and vaccines by supporting countries at regional and global levels [57]. The BMG is a significant partner of COVAX, GAVI, CEPI and WHO to pool donations from wealthy countries. However, COVAX could not deliver its promises until July 2021. This resulted in the low-income nations being short of vaccines. COVAX was set up as the primary avenue for low and middle-income countries with a promise to deliver vaccines which it could not in the initial months due to the complexity of global governance and the market. Therefore, international bodies, scientists, and NGOs had to come forward to demand equitable access to vaccines. In 2021, The BMG joined others in calling for high-income countries to share one billion doses of COVID-19 vaccines with low- and middle-income countries [58].
Key Factors to Strengthen Vaccine Programs
Vaccine Acceptance
Vaccination acceptance rates vary from region to region in high-income countries where the reason for sub-optimal vaccination rates is a lack of acceptance of the vaccines. In low-income countries, however, low vaccine rates are due to access issues.
A study was conducted in 2019 by project AViD (Anthropological Exploration of Facilitators and Barriers to Vaccine Deployment and Administration During Disease Outbreaks) to explore the reasons for vaccine acceptance. The study examined various social, cultural, political, and religious factors affecting vaccine acceptance. It showcased six case studies from low and low-middle-income countries that categorized the factors identified under the ecosystem of anthropological studies policy and systemic levels, as well as local knowledge and perceptions of vaccines. The study helped in the identification of political and economic factors that influence vaccine deployment. It also highlighted how the healthcare system itself could generate vaccine controversy. For example, the impact of the Zika outbreak on public trust through the period of uncertainty of what was causing the microcephaly outbreak in Brazil in 2015 was spreading rumours that an expired batch of vaccines administered by the government was responsible for microcephaly. This pointed to a lingering suspicion of a link between vaccines and microcephaly and resulted in vaccine hesitancy. Also, local perspectives on experimental vaccine deployment during the Ebola epidemic and local knowledge regarding vaccines were responsible for vaccine hesitancy. It is important to understand local cultures and the realities on the ground on how vaccine trials are perceived by people at the local level. Contributions from the social sciences alongside the medical sciences are important for developing and rolling out safe and effective vaccines [59].
During the COVID-19 pandemic, the challenge of vaccine hesitancy in countries was explored— how to rethink health service delivery and how to develop ways to reach out to women, children, and families with quality support and care, thereby, building trust. An analysis was undertaken by the OECD. Its policy paper specified the role of the government in communicating the benefits of the vaccine to the people, ensuring the safe and effective delivery of vaccines, and ensuring efficient and equitable vaccine distribution. Although mass vaccination campaigns are essential, the transparency of the government is also necessary [60]. It is also important to ensure extensive and well managed public engagement. In a study conducted in Jordan, information received from healthcare workers was quoted as the most trusted source of information [61]. To build confidence in vaccines, the US enlisted family doctors and other emissaries in its official plan as these doctors are trusted voices in their communities. The US also announced a new federal website and a phone number to help people find vaccination sites closest to them [62].
Figure 7 shows data collected by the Kaiser Family Foundation (KFF) from the COVID-19 Vaccine Monitor of the US. Potential reasons for getting vaccinated were examined. It was found that transparency can build trust and change the behavior of people towards the acceptance of the vaccine. Increase in COVID-19 cases due to the Delta variant, mandates of various sorts like traveling and attending events, FDA granted full approval for the Pfizer vaccine, social pressure from family and friends, and financial incentives from the employer were among the many motivational reasons for vaccine acceptance [63]. Various other reasons for vaccine acceptance were building confidence, enhancing transparency and undertaking coherent public communications to address misinformation.
Drivers of Vaccine Confidence
A report published by the World Economic Forum analyzed publicly available content from the news media and the social media to capture drivers that correlate with vaccine confidence. The reports discussed how messages on ‘protection offered by the vaccine’ and ‘guarantee of protection by the vaccine’ were the main drivers for positive communications. Moral messages, low trust in the vaccine, and its impact on personal health were the drivers for negative communications. The survey highlighted how simple, positive messages were well received by society. The report showed that the role of the general public was highly effective in building vaccine confidence among friends and family [64, 64]. The all of society approach is needed to protect oneself and the community [64, 64]. The report also found that people rarely distinguished between different vaccines. Other drivers included group identity,Footnote 5 involvement of public figures, and trust in government and other institutions, whether or not people's concerns were valued, and how vaccine confidence, labels, empathy, and risks and benefits.
“It is important to come together and engage in a dialogue to understand public health concerns. Vaccines represent one of the greatest public health advances in modern times. Their role in ending the COVID-19 pandemic depends in large part on understanding how to meet people where they are and listen to and respond to their questions” said Genya Dana, Head of Health and Healthcare at the World Economic Forum.
New Technologies
There are no short-cuts to building vaccine development capacities. Low-income countries are in dire need of adopting advanced technologies. Recent advances in technology have encouraged low-income countries to prepare for such future crises. For example, ‘single use technologies’ and process intensification (lowering the costs of production and improving efficiencies) and ‘fill-finishing’ technology which focuses on manufacturing (the process of putting vaccine ingredients in individual or multi-dose vials in a controlled manner), are important [26]. Apart from investing in vaccine development technologies, data management applications and disease response dashboards are among other systems that countries need to invest in to strengthen and support their healthcare systems.
Vaccine Equity
After years of manufacturing vaccines, we have learned that expanding the geographic distribution of vaccine manufacturing capacity is critical for achieving vaccine equity. It is not a coincidence that countries with domestic capacity to manufacture vaccines have received most of the COVID-19 vaccine doses while those without capacity have been forced to wait. Vaccine manufacturers located in developing countries (or ‘DCVMs’) are also more receptive than large multinational corporations to focus on neglected diseases, especially those diseases that are endemic in those countries and regions like MenAfriVac, an affordable Meningitis A vaccine that came out of a multi-year partnership with PATH and the Serum Institute of India (SII). While bacterial meningitis became rare in much of the world thanks to the vaccine, it still kills tens of thousands of people a year in Sub-Saharan Africa. But since its introduction in 2010, MenAfriVac, the first internationally qualified vaccine developed outside the major multinational pharmaceutical companies and the first vaccine developed specifically for Africa, has effectively ended meningitis as a public health problem there [26].
Learnings from Selected Countries
United States of America
The US faced a major change in addressing the COVID-19 problem from President Donald Trump’s tenure to when President Biden took over office in early 2021 which is when the US COVID response strategy had shifted to vaccination. On January 21, 2021, President Biden released the National Strategy for the COVID-19 Response and Pandemic Preparedness that included seven goals. Goal two promised to mount a safe, effective, comprehensive vaccination campaign sparing no effort to ensure that Americans get vaccinated quickly, effectively, and equitably. President Biden’s efforts focused on making the vaccine widely available by reaching out to those who needed the vaccine shot the most by strengthening the allocation and distribution process. To have a strategy in place meant gaining people's trust, focusing on hard-to-reach and high-risk populations, compensating states and local governments for the cost of administering vaccinations, creating public education campaigns, maintaining transparency, and monitoring vaccine safety by taking the help of the FDA to make available timely vaccine safety and efficacy data to the public [66]. The US followed this strategy to control the spread of the infection.
In the early months of 2021, the US vigorously defended intellectual property rights on vaccines that it had developed. However, in early May, when 50% of its population had received at least one shot, the US announced at the World Trade Organization (WTO) its support for waiving intellectual property protection for COVID-19 vaccines [30]. This delay in waiting for the intellectual property protection is possibly the primary reason why low and lower-middle-income countries are now facing the problem of inequitable access to COVID vaccines.
In June 2021, President Biden, as the leader of the G7 + Plan to Defeat the COVID-19 Pandemic by 2022 welcomed the commitment of G7 and guest countries to provide one billion additional COVID-19 vaccine doses to the world. The US was committed to providing 500 million doses by August 2021 through COVAX, the largest single donation of the vaccine in 12 months. This half a million was in addition to the $2 billion funding that the US provided to COVAX through GAVI and its previous promise of delivering 80 million vaccine doses. The Biden-Harris Administration also promised to provide support for programs worldwide, including in Latin America, Asia, and Africa. Also, through the Quad Vaccine partnership of the US, India, Japan, and Australia, it promised to support vaccine manufacturing in Africa-for Africa and to help in developing regional networks. As part of its transformational disease surveillance and early warning strategy, the US invested 500 million USD to modernize public health data, infrastructure, and established a new Center for Epidemic Forecasting and Outbreak Analytics at the US Centers for Disease Control [67].
“America will be the arsenal of vaccines in our global fight against COVID-19, just as America was the arsenal of democracy in World War II” said Joseph R. Biden, President of the United States [68].
In June 2021, the pace of vaccination fell short of the set target. So, a new phase was launched, shifting vaccination sites to more local settings (doctor’s offices, pharmacies, mobile clinics, and pop-ups in rural areas). The goal in this phase was to vaccinate 70% of adults and those who were resistant to getting vaccinated [62]. Figure 10 shows the progress made by the US to vaccinate its population.
After the CDC declared in August 2021 that 98.8% of infections in the US were due to the Delta variant, the Biden administration started working on new strategies to offer COVID-19 vaccine booster shots to nursing staff. This decision was driven by the data indicating a decline overtime in the vaccines’ ability to protect against new variants [69]. This decision was followed by vaccine mandates when the Bidden administration declared a 75 days period for federal workers to be vaccinated or face termination unless they fell into limited exemption categories [70]. This order also applied to big businesses with more than 100 employees with paid leave by providing an option to either get vaccinated or take weekly tests [71]. Before this mandate, a vaccine communication strategy, with an outreach plan through social media campaigns and advertisements, had been implemented. The campaign was conducted to educate citizens about the efficacy and safety of the vaccine. As part of the strategy, other campaigns, like lotteries for COVID-19 vaccinated residents, sports tickets, tuition fees, cars, beer and even a $1 million cash prize, were undertaken. However, these campaigns were short-lived and yielded no long-term results [71].
In September 2021, when the US was battling the ‘third wave’ of the COVID-19 pandemic, a study conducted by Kiser Family Foundation (KFF) showed that 72% (seven in ten) US adults were at least partially vaccinated. Vaccination in 18–29-year-olds increased by 11 percentage points from July to September 2021. This shift in vaccine acceptance was due to a surge in cases, hospitalizations and deaths due to the Delta variant. The most significant increase in vaccine acceptance and uptake seen from July to September 2021, was mainly due to factors like full FDA approval of the Pfizer vaccine and adoption of other vaccine strategies like vaccine mandates [63, 70]. However, there were differences in the uptake of vaccines related to partisanship, education level, age, and health insurance. Most unvaccinated adults saw the booster dose as a sign that the vaccines were not effective. Among the vaccinated, there was an inclination to take a booster because the FDA and CDC had recommended it. There was much debate about vaccine mandates and boosters in the USA. Different states adopted different vaccination strategies to avoid future crises. People favored the mandate for healthcare workers, school teachers, college students, and federal employees. But there was a perfunctory response among employers to COVID-19 vaccine mandates.
India
India implemented the lockdown too abruptly when the pandemic was declared and was too quick to reopen. Through lockdowns, India could control the initial outbreak better than most countries. India also supported the COVAX Facility and led the DCVMN network of vaccine manufacturers and distributors. However, because it opened too quickly, the second wave took over the country, hitting the nation disastrously. Failing to track how the new variants behaved, the government made quick decisions and changed its vaccination strategy. Earlier, following the WHO recommendation of phase-wise vaccination, India had decided to provide vaccination to all (if you can find a shot). However, because of the over-burdened healthcare systems, the central government made a new condition for individual states to bear the cost of the vaccine. This spontaneous and unplanned change created unrest and an environment where people were on their own until the government later reassured the country. India also had to stop exporting vaccines to low-income countries that it had promised to supply vaccines to.
India started receiving support for vaccines only after the daily caseload exceeded 300,000 during the second wave induced by the Delta variant [72]. This situation was completely different to what it was six months earlier. The Serum Institute and Bharat Biotech, makers of the two effective vaccines distributed in India, pushed up vaccine output to 200 million doses. These two vaccines were in addition to those that were in the pipeline earlier—Biological E, Sputnik, Novavax, and Zydus Cadila. By October 2021, India had successfully administered one billion vaccine doses to the adult population (55% had received one dose and 24% had received two doses) [12].
India, with its international vaccine strategy, sent 66 million doses to 47 countries prior to March 2021. It had to discontinue this supply due to a shortage of vaccines at home and the surge of cases in the summer months [73]. The stepping back of India disrupted vaccination plans around the world—such is the dependence on India of developing countries in Asia. However, later India resumed vaccine supplies to the world kitty with about 5% of its production capacity.
In addition to vaccine production, India played a significant role internationally. For example, along with South Africa, India gave a proposal in 2020 at the WTO ministerial conference about the IP waiver on all COVID interventions. This proposal was crucial in achieving stability in vaccine manufacture and distribution. The US supported the proposal in May 2021 when the call to action was raised against the inequitable distribution of vaccines and vaccine patents. This proposal resulted in the waiver of COVID-19 vaccine technology transfer and manufacture to low-middle-income countries [30].
“Countries including Canada, South Korea, and Bangladesh have shown interest in making COVID vaccines if they can get a patent waiver” said Prof K Srinath Reddy, President, Public Health Foundation of India.
Bhutan
One of the most remarkable and inspirational vaccine strategy stories comes from the least developed country, Bhutan, which has a population of 0.7 million. This tiny Himalayan nation had, by the end of July 2021, vaccinated 90% of its adult population. In just three weeks, it delivered a second vaccine dose to nearly every adult. Bhutan is a good example of science-based policy-making and is a role model for countries facing the challenge of vaccine confidence. Bhutan received support from India during the deployment of the first dose of the vaccine and then received vaccine doses from other countries like the US, Bulgaria, Croatia, China, and Denmark. It received its second dose through COVAX.
Schools, monasteries, and other public buildings were mapped as vaccination sites and digital platforms. And the Bhutan Vaccination System helped to rollout the second vaccine doses. In addition, the Health Ministry itself conducted online conferences in districts and villages to address vaccine-related concerns. The challenge in the healthcare system of the shortage of doctors was met by recalling 50 registered doctors from overseas. The demand for nurses and healthcare workers to manage vaccination sites were met by an ongoing program called ‘Guardians of the Peace.’ A combined effort of solidarity to identify and reach out to the remotest populations and good leadership were the hallmarks of Bhutan’s vaccine rollout. As a role model for low and middle-income countries, Bhutan showed us how to achieve equitable vaccine rollout [74].
Kenya
Vaccinating people for COVID-19 has been a challenge when vaccinating hard-to-reach communities. Several strategies were adopted worldwide to enhance vaccine acceptance among these communities and to check if there were enough vaccine supplies. Kenya adopted one such vaccination strategy to reach nomadic herders which was the most challenging community to reach. The authorities planned to reach out to 250,000 people of these cross-border communities and encourage them to get a jab to boost the uptake of the COVID vaccine. They offered the COVID-19 vaccine and livestock vaccination at the same time [75].
Israel
Israel was one of the first few countries that initiated a national campaign to vaccinate its population against COVID-19 in December 2020. With a population size of 9.1 million and a population density of 400 per square kilometer, Israel rapidly rolled out its vaccination campaign.
During the time-line of one year (December 2020 to December 2021), Israel had successfully administered 10 million vaccine doses in a quarter with 54% of its entire population being fully vaccinated. A well-developed health system that includes advanced information technology and logistical capacity of community-based healthcare providers, well trained and salaried nurses, cooperation between central and state governments, health plans, hospitals, and emergency care providers along with an effective existing system for implementing prompt services to address large-scale national emergencies, was in place in Israel. The existing system was able to prioritize, allocate, and document its vaccine eligible population and to vaccinate them [76, 77]. It already had a robust system in place. There were other factors specific to the COVID-19 vaccination effort that included: government funding for vaccine purchase and distribution, timely contracting for vaccines required for Israel’s population, determined priority, distribution process, creative cold storage, demands for the Pfizer-BioNTeach COVID-19 vaccines, and well-tailored outreach efforts to increase vaccine acceptance.
From a 7-day moving average of new COVID-19 cases of 149 per day in April 2021, there is an increase to a 7,320 per day 7- day moving average in August 2021, Israel saw a dramatic change in the COVID-19 vaccination response rate. The number of COVID-19 cases peaked in September 2021 despite 60% of the population being fully vaccinated. This was the result of a third wave fueled by the Delta variant. The share of Delta-positive sequences in Israel increased from 13% in the first week of June to 87% in mid-August indicating that the new cases were driven by the Delta variant [78]. The rise in cases among the fully vaccinated showed that the efficacy of the vaccine in preventing infections against the Delta variant had reduced but the share of severely ill patients and fatalities was recorded to be lower among the fully vaccinated. This data also indicated that the vaccine continued to protect people against critical infection, sever illness, and death. Considering the existing data, Israel adopted the strategy of administering a third dose to people over 60 years of age who had received their second shot at least five months earlier. Israel’s Ministry of Health used aggregated data from the national SARS-CoV-2 surveillance, vaccination program dataset, and other sources such as research studies that compared disease incidence in vaccinated and unvaccinated people. This data helped to develop efficacy estimates of the Pfizer vaccine [76]. The 7-day moving average reduced to 457 per day in the month of November 2021 with over 64% of the population being fully vaccinated and 42% having received COVID-19 booster doses.
In a successful vaccination campaign, continuous sharing of data through a full bodied platform to examine vaccine effectiveness and the impact of high vaccine coverage, in real-world conditions helped the scientific community to undertake parallel studies. This continuous data sharing helped to develop an understanding of how demographic and socioeconomic characteristics are significant predictors of vaccination behavior and how this changed when the new Variant of Concern was detected in the country in August 2021. These studies also revealed that a lack of confidence in COVID-19 vaccines is a major factor in vaccine hesitancy [79].
United Kingdom
The United Kingdom (UK) with a population of 40 million and 54 per square kilometer population density, was the first country in the world to undertake a COVID-19 vaccination program by approving the use of Pfizer/BioNTech vaccine and starting inoculations on December 8, 2020, and then of AstraZeneca by December 30, 2020. During that time the 7-day moving average was around 30,000 new COVID-19 cases per day, which soon decreased to 5,000 [7] new cases per day by March 2021 when phase 1 of vaccination was completed. The vaccine was offered to people based on advice from the Joint Committee on Vaccination and Immunization (JCVI). The administration of the vaccine was divided into phase 1 and phase 2 with group numbers from 1 to 12 categorized based on descending age orders and priorities. Vaccination for 12–15 years old children was rolled out on September 20, 2021 [81, 82].
Based on the JCVI recommendations, in August 2021, the UK government started a ‘third primary dose’ of vaccination for individuals aged 12 and over who were severely immune suppressed when or shortly after they received their first or second doses. This primary third dose was different from the booster dose that was started in September 2021 for the phase 1 group and in November 2021 onwards for phase 2 and other groups.
Two doses of the Pfizer/BioNTech and AstraZeneca vaccine were estimated to be 96% and 92% effective, respectively against hospitalization with the Delta variant. Even after providing four vaccines for use: Pfizer-BioNTech, Oxford-AstraZeneca, Moderna, and Janssen and the high rate of COVID vaccination, the UK showed an unusual trend of new COVID cases recorded on a daily basis since July 2021. Until November 2021, the UK had vaccinated more than 8 in 10 individuals aged 18 and over with both doses of the vaccine and around 6 in 10 individuals aged 50 and over with a booster or third dose. But the 7-day moving average of new COVID cases reached as high as 40,000 per day with the Omicron strain which spreads faster. It was also found that people who had already received two doses of the COVID vaccine were being hospitalized [80,81,82]. This raised concerns. It looked like a race between ‘the virus and the vaccine’[83].
UK, with an average rate of 400,000 vaccine doses administered per day, placed a spotlight on the risks and benefits of expanding the vaccination program in an effort to help other nations and make vaccines available in poorer countries or to focus on the UK epidemic. Other options like providing resources and funding to COVAX, the global vaccine sharing initiative, supporting technology transfer agreements to domestic manufactures, and scaling up vaccine production helped to ensure that at least 10% of the world was vaccinated and also prevented the emergence of new strains as recommended by WHO [84].
Japan
A country with a population of 126 million and a population density of 347 per square kilometer, organized one of the most effective COVID-19 vaccination programs. But Japan delayed its COVID-19 vaccination campaign by two months compared to several other developed countries that started their COVID-19 vaccination campaign in December 2020 as soon as COVID-19 vaccines became available.
The lag in the rollout can be attributed to: (1) the delay in the COVID-19 vaccines regulatory approval process that required domestic clinical trials. Japan required more clinical tests than other countries for the vaccine to be deemed safe [85, 86]. It was also due to the smaller number of patients with COVID-19 recorded initially in the country, which were less than the number required to register into international clinical trials to prove vaccine efficacy. The delay in the country's own review process was due to changes in the regulations for vaccine approval that were considered only after Japan’s program was criticized. (2) Japan also experienced a delay in vaccine importation. (3) In Japan the vaccine rollout system was insufficient for achieving mass vaccination and ensuring other legal bindings [87].
Japan rolled out its vaccination program in the middle of February 2021 when the 7-day moving average of new COVID cases was recorded to be 1,500 per day. After the slow rollout of COVID vaccines with only 3% of its population being fully vaccinated at the start of June 2021, the country was under pressure to reduce the infection case load before the Summer Olympics when the number of new COVID cases was recorded to be as high as 3,553 cases per day [7] by July 2021. However, Japanese doctors administered more than one million doses a day throughout the summer and the country fully vaccinated 45% of the population by the end of August 2021 with three vaccines AstraZeneca, Pfizer, and Moderna [88]. This vaccination rate was achieved while the Summer Olympics 2021 were ongoing. By November 2021, Japan fully vaccinated 77% of its population and reported 90 new COVID cases per day [7].
South Africa
South Africa experienced the maximum number of COVID waves with the fourth wave of the Omicron variant reported in December 2021. This African country, with a population density of 47 per square kilometer, has 20% of its 60 million population with waning immunity due to HIV. It is one of the most vulnerable countries [89]. So, to meet the demand, the government being the sole responsible body for sourcing, distributing, and overseeing the rollout of the vaccine, adopted various strategies to vaccinate its population. The international body COVAX played a major role in arranging for the vaccine.
Drug and vaccine manufacturers have always preferred bigger markets as testing hubs to avoid the expense and uncertainties of testing products, which is why less than 3% of clinical trials were implemented in Africa. The constant mutation of the COVID virus made it evident to the world that manufacturers cannot afford to wait years to test the efficiency of vaccines in poor countries. It was due to the efforts of Shabir Madhi, who approached the Oxford team and Novavax to conduct clinical trials in South Africa to highlight how different socioeconomic and health conditions can change the vaccine’s performance. But he faced many challenges even after getting funding from the Bill and Melinda Gates Foundation to run the vaccine trials. This was due to lack of resources like cold freezers and backup generators and a shortage of a trained workforce. Despite these challenges, along with his team, he somehow managed to conduct clinical trials with limited resources. This promoted South Africa as the epicenter of clinical trials and as an important local vaccine producer [89]. The goal of conducting clinical trials in South Africa was to later leverage deals with vaccine manufacturers to provide vaccines to South Africa. However, this really did not happen.
The daily confirmed COVID-19 new deaths that had peaked at 292 during the first wave, rose to 577 deaths in the second wave and then declined to 420 deaths during the third wave. The fourth wave again picked up exactly when the third reached its tail. In a span of two weeks, South Africa experienced a significant rise in new COVID cases from 1,200 in the last week of November 2021 to 16,000 new COVID cases per day as per [7] in the last week of December 2021. This was because of the low vaccination rate in South Africa with only 42 doses per 100 people. This rate was even lower in other African countries [90]. Due to its reliance on a combination of bilateral deals, donations, and the COVAX vaccine sharing scheme, the country struggled to get supplies until August 2021. It was not just the uneven supply of the vaccine, but the country also faced the issues of misinformation or a lack of good information about the safety of the vaccine [90]. Initial issues were related to vaccine deployment through the central management approach using an electronic data system that was later abandoned as it made vaccines less accessible.
Comirnaty and Johnson & Johnson’s JNJ-78436735 are two authorized COVID-19 vaccines in use in South Africa with enough supply. But the challenge was to deploy them. Adopting a new approach of walk-in vaccinations, South Africa did fairly well but challenges remain because most of its population is living with HIV infection.
The country has a long-term agenda which is to increase its own vaccine production capacity from the current 1% rate of production to 60% that Africa needs. COVID-19 vaccine trials will definitely serve as an important starting point to generate future opportunities [91].
Concluding Comments
The vaccine for COVID-19 has been the fastest vaccine ever developed and deployed. Continuous efforts by the scientific community, international organizations, and governments made this possible. Studying the vaccination rates of densely populated countries and analyzing COVID-19 vaccination strategies of these countries provides a clear picture of progress made so far. By using international frameworks and vaccination strategies for COVID-19, this chapter underscores that there are substantial challenges in the development, procurement, and supply of COVID vaccines and their impact on vaccination in low- and middle-income countries. Patents for COVID-19 vaccines, waning immunity, the need for booster doses, and surplus vaccine stocks in Western countries are the factors responsible for vaccine inequity. Despite the efforts of international organizations and coalitions like CEPI, WHO, PAHO, GAVI, COVAX, and G7, manufacturing vaccines and making them available equitably has been a difficult goal to achieve. The lack of equity has resulted in high vaccination rates in high-income countries and uncertainty of vaccine access in the rest of the world. Unequal vaccine distribution is one of the main reasons for the relapse in COVID-19 infection even in countries which have 75% fully vaccinated populations.
A review of data on COVID-19 infections and vaccination rates draws attention to factors that strengthen COVID-19 vaccination strategies. This review provides an understanding of interlinkages among various factors. For example, technology is important for keeping records, reaching out and vaccinating larger populations, and ensuring transparency. Data transparency, in turn, is necessary for building vaccine confidence among the people and, thereby, increasing vaccine acceptance.
While fully vaccinating everyone against COVID-19 is necessary for controlling the pandemic, equal distribution of the COVID-19 vaccine globally is still a challenge that needs to be addressed. Equity can be achieved by developing stronger international frameworks and actively engaging high-income countries in donating vaccines to the developing world.
Notes
- 1.
Variants of Concern are SARS-CoV-2 variants with the following concerns Increase in transmissibility or detrimental change in COVID-19 epidemiology; or increase in virulence or change in clinical disease presentation; or decrease in the effectiveness of public health and social measures or available diagnostics, vaccines, and therapeutics.
- 2.
COVAX, a COVID-19 vaccine portfolio with nine CEPI-supported candidate vaccines has a goal to bring the pandemic under control via equitable access to COVID-19 vaccines.
- 3.
The use of the term ‘public good’ in global health means a good that should be available universally because of its critical importance to health.
- 4.
Pockmarks are deep scars on the skin that do not usually go away on their own. They are often caused by severe acne but can also be the result of skin infections, chickenpox, and smallpox.
- 5.
Group identity here refers to the groups with which an individual self-identifies. This includes where the group sits politically, societally, and what defines its values and beliefs.
References
Worldometer. COVID Live—Coronavirus statistics. Worldometer. https://www.worldometers.info/coronavirus/
World Health Organization. Status of COVID-19 vaccines within World Health Organization emergency use license evaluation process. Extranet- World Health Organization. 2021 Sep 29. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_07July2022.pdf
Pan America Health Organization. Pharmacovigilance for COVID-19 vaccines: Catalogue. Pharmacovigilance- Pan America Health Organization. https://covid-19pharmacovigilance.paho.org/
Maxmen A. The fight to manufacture COVID vaccines in lower-income countries. Nature. 2021 Sep 15. https://www.nature.com/articles/d41586-021-02383-z
Penn M. For much of the world, the COVID fight is just beginning. Duke Global Health Institute. 2021 Oct 13. https://globalhealth.duke.edu/news/much-world-covid-fight-just-beginning
Henley J, Kassam A, Connolly K, Giuffrida A. Which countries are vaccinating minors against Covid? The Guardian. 2021 Aug 4. https://www.theguardian.com/world/2021/aug/04/which-countries-are-vaccinating-minors-against-covid
Rich countries with 53% population received 83% of world’s vaccines: WHO chief. mint. 2021 May 11. https://www.livemint.com/news/india/rich-countries-with-53-population-received-83-world-s-vaccines-who-chief-11620712139742.html
Worldometer. Population by Country (2022) - Worldometer. https://www.worldometers.info/world-population/population-by-country/
The New York Times. Covid world vaccination tracker - The New York Times. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html
Callaway E. Delta coronavirus variant: Scientists brace for impact. Nature. 2021 Jun 22; 595(7865): 17–18. https://www.nature.com/articles/d41586-021-01696-3
Business Standard News. Indian Covid variant B.1.617 detected in 44 countries, says World Health Organization. Business Standard News. 2021 May 12. https://www.business-standard.com/article/current-affairs/indian-covid-variant-b-1-617-detected-in-44-countries-says-who-121051200167_1.html
Business Line. Vaccine diplomacy returns. Business Line. 2021 Sep 27. https://www.thehindubusinessline.com/opinion/editorial/it-is-just-as-well-that-india-is-once-again-set-to-produce-vaccines-for-the-rest-of-the-world/article36698983.ece
Brown G. Equitable Distribution: Universal vax - The global north’s great test. dtNext. 2021 Sep 25. https://www.dtnext.in/world/2021/09/25/equitable-distribution-universal-vax--the-global-norths-great-test
Loftus P, Hopkins JS. Pfizer-BioNTech Covid-19 vaccine for young kids satisfied fda criteria, agency says. The Wall Street Journal. 2021 Oct 22. https://www.wsj.com/articles/fda-review-of-pfizer-covid-19-vaccine-for-kids-expected-11634900401
Department of Health and Social Care. Young people aged 12 to 15 to be offered a COVID-19 vaccine. Gov.UK. 2021 Sep 13. https://www.gov.uk/government/news/young-people-aged-12-to-15-to-be-offered-a-covid-19-vaccine
Reuters. These countries are vaccinating children against COVID-19. Canadian Television News. 2021 Oct 19. https://www.ctvnews.ca/health/coronavirus/these-countries-are-vaccinating-children-against-covid-19-1.5629655
French C. Kids and vaccines: Half of Canadian parents eager to vaccinate. Canadian Television News. https://www.ctvnews.ca/health/coronavirus/half-of-canadian-parents-would-vaccinate-their-5-11-year-old-asap-survey-1.5627483 Revised. Half of Canadian parents would vaccinate their 5–11 year old ASAP: survey.
World Health Organization. 172 countries and multiple candidate vaccines engaged in COVID-19 vaccine Global Access Facility. World Health Organization. 2020 Aug 24. https://www.who.int/news/item/24-08-2020-172-countries-and-multiple-candidate-vaccines-engaged-in-covid-19-vaccine-global-access-facility
Airfinity Home of New Science. COVID-19 vaccine expiry forecast for 2021 and 2022. Airfinity Home of New Science. 2021 Sep 20. https://www.airfinity.com/reports/covid-vaccine-expiry-forecast-2021-2022
Holder J. Tracking Coronavirus vaccinations around the world. The New York Times. 2021 Jan 29. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html
World Health Organization. WHO SAGE roadmap for prioritizing uses of COVID-19 vaccines in the context of limited supply. World Health Organization. 2020 Nov 13. https://apps.who.int/iris/handle/10665/342917
World Health Organization. WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. World Health Organization. 2020 Sep 14. https://www.who.int/publications/i/item/who-sage-values-framework-for-the-allocation-and-prioritization-of-covid-19-vaccination
D'Souza G, Dowdy D. Rethinking Herd Immunity and the Covid-19 Response End Game. Johns Hopkins Bloomberg School of Public Health. 2021 Sep 13. https://publichealth.jhu.edu/2021/what-is-herd-immunity-and-how-can-we-achieve-it-with-covid-19
McCallum K. Herd immunity: How many people need to get the COVID-19 vaccine?. Houston Methodist On Health. https://www.houstonmethodist.org/blog/articles/2020/dec/herd-immunity-how-many-people-need-to-get-the-covid-19-vaccine/
Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, Macdonald B, Beltekian D, Roser M. Coronavirus Pandemic (COVID-19). Our World Data. 2020 Mar 5. https://ourworldindata.org/coronavirus
Zaidi A. Geographically distributed manufacturing capacity is needed for improved global health security. Bill, Melinda Gates Foundation. 2021 Jul 28. https://www.gatesfoundation.org/ideas/articles/covid19-vaccine-geographic-distribution
Arita I, Wickett J, Fenner F. Impact of population density on immunization programmes. The Journal of Hygiene (London). 1986 Jun; 96(3): 459–466. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2129696/
Tartar A, Brown KV, Randall T. Growing gaps in U.S. vaccination rates show regions at risk. Bloomberg Quint. 2021 Jun 29. https://www.bloomberg.com/news/articles/2021-06-29/growing-gaps-in-u-s-vaccination-rates-show-regions-at-risk
Vaitilingam R. Vaccines for developing countries: Views of leading economists on patent waivers and vaccinating the world. Centre for Economic Policy Research. 2021 May 22. https://cepr.org/voxeu/columns/vaccines-developing-countries-views-leading-economists-patent-waivers-and-vaccinating
Magazine A, Raghavan P. Explained: Intellectual property waiver for Covid-19 vaccines | Explained News, the Indian Express. 2021 May 11. https://indianexpress.com/article/explained/explained-ip-waiver-for-covid-vaccines-7304992/
Organizacion Panamericana de la Salud. Consolidated regional and global information on adverse events following immunization (AEFI) against covid-19 and other updates. Pan American Health Organization. https://covid-19pharmacovigilance.paho.org/img/recursos/6154e88fc542faac43a66b4e8.pdf
Global Health Summit Scientific Expert Panel. Global health summit. Global Health Summit. 2021 May 21. https://global-health-summit.europa.eu/panel-scientific-experts_en
London School of Hygiene and Tropical Medicine. Investment in science and innovation needed to tackle COVID-19 and future health threats while ensuring equitable access - expert panel. London School of Hygiene and Tropical Medicine. 2021 May 21. https://www.lshtm.ac.uk/newsevents/news/2021/investment-science-and-innovation-needed-tackle-covid-19-and-future-health
International Federation of Pharmaceutical Manufacturers and Associations. Momentum of COVID-19 vaccine manufacturing scale up sufficient for step change in distribution. International Federation of Pharmaceutical Manufacturers and Associations. 2021 Sep 07. https://www.ifpma.org/resource-centre/momentum-of-covid-19-vaccine-manufacturing-production-scale-up-is-now-sufficient-for-step-change-in-distribution-and-opens-way-for-urgent-political-leadership-and-country-preparedness/
International Federation of Pharmaceutical Manufacturers and Associations. Five steps to urgently advance COVID-19 vaccine equity. International Federation of Pharmaceutical Manufacturers and Associations. 2021 May 19. https://www.ifpma.org/resource-centre/five-steps-to-urgently-advance-covid-19-vaccine-equity/
International Federation of Pharmaceutical Manufacturers and Associations. The biopharmaceutical industry is at the forefront of the fight against the COVID-19 pandemic. International Federation of Pharmaceutical Manufacturers and Associations. https://www.ifpma.org/covid19/
Airfinity Home of New Science. September 2021 snap shot COVID-19 data. Airfinity Home of New Science. 2021 Sep 07. https://www.ifpma.org/wp-content/uploads/2021/09/Airfinity_September_2021_Snapshot_COVID-19_Data.pdf
Airfinity Insights - Global press release: More than a billion available stock of Western COVID-19 vaccines by the end of 2021. Airfinity. 2021 Sep 05. https://www.airfinity.com/articles/more-than-a-billion-available-stock-of-western-covid-19-vaccines-by-the-end
Airfinity. COVID-19 Vaccine Production and surplus doses. Airfinity. 2021 May 19. https://www.ifpma.org/wp-content/uploads/2021/05/airfinity_production_19.05.2021.pdf
Airfinity. COVID-19 vaccine expiry forecast for 2021 and 2022. Airfinity. 2021 Sep 20. https://assets.ctfassets.net/poihmvxzgivq/6pooxWkcaF1bHmlP7M5dtP/d4bdc2c7707f4a4ad56b7e967063fa84/Vaccine_expiry_forecast_airfinity_21.09.20.pdf
Gates B. A three-part plan to eliminate COVID-19. Bill, Melinda Gates Foundation. https://asia.nikkei.com/Opinion/A-three-part-plan-to-eliminate-COVID-19
The Global Alliance for Vaccines and Immunizations. Gulf countries unite to support COVAX. The Global Alliance for Vaccines and Immunizations. 2021 Oct 06. https://www.gavi.org/news/media-room/gulf-countries-unite-support-covax
The Global Alliance for Vaccines and Immunizations. COVAX explained. The Global Alliance for Vaccines and Immunizations. 2020 Sep 03. https://www.gavi.org/vaccineswork/covax-explained
COVAX Manufacturing Task Force. COVAX Manufacturing Task Force to tackle vaccine supply challenges. Coalition for Epidemic Preparedness Innovations. 2021 May 14. https://cepi.net/news_cepi/covax-manufacturing-task-force/
The Global Alliance for Vaccines and Immunizations. COVAX vaccine roll-out. The Global Alliance for Vaccines and Immunizations. 2022 Jan 17. https://www.gavi.org/covax-vaccine-roll-out
The Global Alliance for Vaccines and Immunizations. The COVAX Humanitarian Buffer Explained. The Global Alliance for Vaccines and Immunizations. 2021 Mar 30. https://www.gavi.org/vaccineswork/covax-humanitarian-buffer-explained
Coalition for Epidemic Preparedness Innovations. Investment Case. Coalition for Epidemic Preparedness Innovations. https://endpandemics.cepi.net/
World Health Organization. COVID-19 vaccine country readiness and delivery. World Health Organization. https://www.who.int/initiatives/act-accelerator/covax/covid-19-vaccine-country-readiness-and-delivery
Pan American Health Organization. Who We Are - PAHO/WHO. Pan American Health Organization. https://www.paho.org/en/who-we-are
World Health Organization. PAHO paving the way for COVID-19 vaccination in the Americas. World Health Organization. 2021 Jun 18. https://www.paho.org/en/stories/paho-paving-way-covid-19-vaccination-americas#:~:text=18%20June%202021%20%2D%20Following%20decades,COVAX%20Facility%20in%20the%20Americas.
Wikipedia. Group of Seven. In. Wikipedia. https://en.wikipedia.org/wiki/Group_of_Seven_(artists)
European Council. G7 leaders’ Statement on COVID-19. European Council of the European Union. 2020 Mar 16. https://www.consilium.europa.eu/en/press/press-releases/2020/03/16/g7-leaders-statement-on-covid-19/
Wintour P. UK falling behind most G7 countries in sharing Covid vaccines, figures show. The Guardian. 2021 Oct 24. https://www.theguardian.com/world/2021/oct/24/uk-falling-behind-most-g7-countries-in-sharing-covid-vaccines-figures-show
Lee J, Morton B. G7: World leaders promise one billion Covid vaccine doses for poorer nations. British Broadcasting Cooperation News. 2021 Jun 13. https://www.bbc.com/news/uk-57461640
United Nations Children's Fund. Getting COVID-19 vaccines to West and Central Africa | UNICEF Supply Division. United Nations Children's Fund. https://www.unicef.org/supply/stories/getting-covid-19-vaccines-west-and-central-africa
United Nations Children's Fund. Z Zurich Foundation campaign aims to help UNICEF deliver over 2.5 million COVID-19 vaccine doses. United Nations Children's Fund. 2021 Jul 13. https://www.unicef.org/partnerships/z-zurich-foundation-campaign-aims-help-unicef-deliver-covid-19-vaccines
Ghosh G. Global response needed to end COVID-19 and prevent pandemics. Bill & Melinda Gates Foundation. 2021 Sep 21. https://www.gatesfoundation.org/ideas/articles/global-response-needed-to-end-covid-19
Theopold N. Why we’re calling for sharing 1 billion COVID-19 vaccine doses | Bill & Melinda Gates Foundation - Bill & Melinda Gates Foundation. 2021 May 28. https://www.gatesfoundation.org/ideas/articles/covid19-vaccine-doses-covax Revised. Sharing COVID-19 vaccines can help save lives.
London School of Hygiene and Tropical Medicine. Vaccine trials must engage with communities or risk failure, say social scientists. London School of Hygiene and Tropical Medicine News. 2020 Oct 09. https://www.lshtm.ac.uk/newsevents/news/2020/vaccine-trials-must-engage-communities-or-risk-failure-say-social-scientists
Organisation for Economic Co-operation and Development. Enhancing public trust in COVID-19 vaccination: The role of governments. Organisation for Economic Co-operation and Development. 2021 May 10. https://www.oecd.org/coronavirus/policy-responses/enhancing-public-trust-in-covid-19-vaccination-the-role-of-governments-eae0ec5a/
El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ. Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. Public Library of Science. ONE. 2021 Apr 23; 16(4). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8064595/
LaFraniere S, Weiland N. Biden announces a strategy change as the pace of vaccination slows. The New York Times. 2021 May 4. https://www.nytimes.com/2021/05/04/us/biden-covid-vaccine-strategy.html
Kaiser Family Foundation. KFF COVID-19 Vaccine Monitor: November 2021. Kaiser Family Foundation. 2021 Dec 02. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-november-2021/
London School of Hygiene and Tropical Medicine. Analysis of online conversations paints a picture of vaccine confidence. London School of Hygiene and Tropical Medicine. 2021 May 25. https://www.lshtm.ac.uk/newsevents/news/2021/analysis-online-conversations-paints-picture-vaccine-confidence
World Economic Forum. How to Build Trust in Vaccines: Understanding the drivers of vaccine confidence. World Economic Forum. 2021 May 25. https://www3.weforum.org/docs/WEF_How_to_Build_Trust_in_Vaccines_2021.pdf
White House. National strategy for the COVID-19 responses and pandemic preparedness. White House. 2021 Jan. https://www.whitehouse.gov/covidplan/
White House. Fact sheet: United States and G7+ Plan to Defeat the COVID-19 Pandemic in 2022 and Prevent the Next Pandemic. The White House. 2021 Jun 11. https://www.whitehouse.gov/briefing-room/statements-releases/2021/06/11/fact-sheet-united-states-and-g7-plan-to-defeat-the-covid-19-pandemic-in-2022-and-prevent-the-next-pandemic/
United States Department of State. COVID-19 Recovery. United States Department of State. https://www.state.gov/covid-19-recovery/
McGinley L, Pager T, Knowles H, Suliman A, Pietsch B, Shammas B, Linskey A, Beachum L. Biden administration to offer vaccine booster shots beginning Sept. 20, require vaccinations for nursing home staff. The Washington Post. 2021 Aug 18. https://www.washingtonpost.com/nation/2021/08/18/covid-delta-variant-live-updates/
Mason J, Aboulenein A, Hunnicutt T. Attacking anti-vaccine movement, Joe Biden mandates widespread COVID shots, tests. Reuters. 2021 Sep 10. https://www.reuters.com/world/us/biden-deliver-six-step-plan-covid-19-pandemic-2021-09-09/
Express Web Desk. Explained: What Joe Biden’s vaccine mandate means for US. The Indian Express. 2021 Sep 10. https://indianexpress.com/article/explained/explained-joe-biden-coronavirus-vaccine-mandate-united-states-7500716/
Banerjee A, Duflo E. Opinion. India’s Problem Is Now the World’s Problem. The New York Times. 2021 May 6. https://www.nytimes.com/2021/05/06/opinion/covid-india-crisis.html
Airfinity. Insights - Halting India’s vaccine exports: The fallout. Airfinity. 2022 Jan 21. https://www.airfinity.com/insights
The Global Alliance for Vaccines and Immunizations. What the world can learn from Bhutan’s rapid COVID vaccine rollout. The Global Alliance for Vaccines and Immunizations. 2021 Sep 28. https://www.gavi.org/vaccineswork/what-world-can-learn-bhutans-rapid-covid-vaccine-rollout
The Global Alliance for Vaccines and Immunizations. How will vaccinating camels boost uptake of COVID-19 vaccines? The Global Alliance for Vaccines and Immunizations. 2021 Oct 05. https://www.gavi.org/vaccineswork/how-will-vaccinating-camels-boost-uptake-covid-19-vaccines
Leshem E, Wilder-Smith. COVID-19 vaccine impact in Israel and a way out of the pandemic. The Lancet. 2021 May 05. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2821%2901018-7/fulltext
Rosen B, Waitzberg R, Israeli A. Israel’s rapid rollout of vaccinations for COVID-19. Israel Journal of Health Policy Research. 2021 Jan 26. https://ijhpr.biomedcentral.com/articles/https://doi.org/10.1186/s13584-021-00440-6
The Hindu. Data | Israel’s recent COVID-19 spike explained. The Hindu. 2021 Sep 11. https://www.thehindu.com/data/data-israels-recent-covid-19-spike-explained/article36402195.ece
Heller O, Shlomo Y, Chun Y, Acri M, Grinstein-Weiss G. The game is not yet over, and vaccines still matter: Lessons from a study on Israel’s COVID-19 vaccination. Brookings. 2021 Sep 13. https://www.brookings.edu/blog/up-front/2021/09/13/the-game-is-not-yet-over-and-vaccines-still-matter-lessons-from-a-study-on-israels-covid-19-vaccination/
National Health Service, England. COVID-19 vaccination statistics. National Health Service, England. 2021 Nov 28. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-vaccinations/
Statista. UK: COVID-19 vaccine numbers by dose. Statista. 2022 March 23. https://www.statista.com/statistics/1194668/uk-covid-19-vaccines-administered/
Srinivasan C. Omicron cases in UK: Hospitalisations, deaths to drastically increase - Sajid Javid on Omicron. 2021 Dec 24. https://www.ndtv.com/world-news/coronavirus-omicron-cases-hospitalisations-deaths-to-drastically-increase-sajid-javid-on-omicron-2650058
The Visual and Data Journalism Team. Covid vaccine: How many people are vaccinated in the UK? British Broadcasting Corporation News. 2022 Mar 04. https://www.bbc.com/news/health-55274833
Davis N. UK faces difficult choices on future Covid vaccination strategy. The Guardian. 2021 Aug 5. https://www.theguardian.com/society/2021/aug/05/uk-face-difficult-choice-future-covid-vaccination-strategy-children-booster-doses-global-effort-save-lives
Essig B. Why Japan took so long to start Covid-19 vaccinations, even with the Olympics looming. Cable News Network. 2021 Feb 28. https://edition.cnn.com/2021/02/26/asia/japan-covid-vaccination-program-intl-hnk-dst/index.html
Steen E. Here’s the tentative timeline of Japan’s Covid-19 vaccination programme. Time Out Tokyo. 2021 Sep 21. https://www.timeout.com/tokyo/news/heres-the-tentative-timeline-of-japans-covid-19-vaccination-programme-012021
Kosaka M, Hashimoto T, Ozaki A, Tanimoto T, Kami M. Delayed COVID-19 vaccine roll-out in Japan. The Lancet. 2021 Jun 19; 397(10292): 2334–2335. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01220-4/fulltext
Gannon J, Kato K. Japan’s COVID-19 vaccination drive success is an opportunity for global leadership. The Diplomat. 2021 Aug 31. https://thediplomat.com/2021/08/japans-covid-19-vaccination-drive-success-is-an-opportunity-for-global-leadership/
Castaneda R (2021) Covid-19 South Africa: in conversation vaccine expert Shabir Madhi. Clinical Trials Arena. 2021 Oct 28. https://www.clinicaltrialsarena.com/analysis/covid-19-south-africa/
Schraer R, Horton J (2021) New Omicron variant: Are low vaccination rates in South Africa a factor? British Broadcasting Corporation. 2021 Dec 3. https://www.bbc.com/news/59462647
Deutsche Gesellschaft für Internationale Zusammenarbeit. ‘South Africa can play an important role in local vaccine production’. Deutsche Gesellschaft für Internationale Zusammenarbeit. Accessed 2021 Sep 13. https://www.giz.de/en/mediacenter/100897.html
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Pathak, D. (2023). COVID-19 Vaccination: A Necessitated Drive Becoming an Unsolved Puzzle. In: Pachauri, S., Pachauri, A. (eds) Global Perspectives of COVID-19 Pandemic on Health, Education, and Role of Media. Springer, Singapore. https://doi.org/10.1007/978-981-99-1106-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-99-1106-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1105-9
Online ISBN: 978-981-99-1106-6
eBook Packages: Social SciencesSocial Sciences (R0)