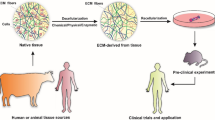
Abstract
Since the development of tissue engineering, many mature decellularization schemes have been formed for different organs. The purpose of decellularization is to remove immunogenic components (such as alpha-Gal epitopes) and cellular components from the organ while retaining the inherent characteristics of natural extracellular matrix (ECM). The efficiency of removal of cells from the organs depends on the methods of decellularization. The commonly used decellularization methods can be divided into physical methods, chemical methods, and enzymatic hydrolysis methods according to the different reagents or conditions used. In the process of whole-organ decellularization, perfusion decellularization is currently the most widely used method and one of the safest and most effective decellularization methods. Each of these treatments affects the biochemical composition, tissue ultrastructure, mechanical behavior of the remaining ECM scaffold and detergent residue. This chapter presents an overview of the decellularization of different whole organs, including heart, lung, liver, kidney, pancreas, intestine, and others. Intended as a broad-ranging reference, this chapter will be of value for those concerned with the study of tissue engineering.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9.
Gupta SK, Mishra NC, Dhasmana A. Decellularization Methods for Scaffold Fabrication. Methods Mol Biol. 2018;1577:1–10.
Guler S, Aslan B, Hosseinian P, Aydin HM. Supercritical carbon dioxide-assisted Decellularization of aorta and cornea. Tissue Eng Part C Methods. 2017;23:540–7.
Struecker B, Butter A, Hillebrandt K, Polenz D, Reutzel-Selke A, Tang P, et al. Improved rat liver decellularization by arterial perfusion under oscillating pressure conditions. J Tissue Eng Regen Med. 2017;11:531–41.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43.
Lü WD, Zhang L, Wu CL, Liu ZG, Lei GY, Liu J, et al. Development of an acellular tumor extracellular matrix as a three-dimensional scaffold for tumor engineering. PLoS One. 2014;9:e103672.
Morris AH, Chang J, Kyriakides TR. Inadequate processing of decellularized dermal matrix reduces cell viability in vitro and increases apoptosis and acute inflammation in vivo. BioRes Open Access. 2016;5:177–87.
Jamur MC, Oliver C. Permeabilization of cell membranes. Methods Mol Biol. 2010;588:63–6.
Simões IN, Vale P, Soker S, Atala A, Keller D, Noiva R, et al. Acellular urethra bioscaffold: decellularization of whole urethras for tissue engineering applications. Sci Rep. 2017;7:1–13.
Liem PH, Morimoto N, Ito R, Kawai K, Suzuki S. Autologous skin reconstruction by combining epidermis and acellular dermal matrix tissue derived from the skin of giant congenital melanocytic nevi. J Artif Organs. 2013;16:332–42.
Hellström M, El-Akouri RR, Sihlbom C, Olsson BM, Lengqvist J, Bäckdahl H, et al. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014;10:5034–42.
Rodi PM, Gianello MB, Corregido MC, Gennaro AM. Comparative study of the interaction of CHAPS and triton X-100 with the erythrocyte membrane. Biochim Biophys Acta. 2014;1838:859–66.
Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM, et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2011;17:411–21.
Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–17.
Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756–64.
Hillebrandt K, Polenz D, Butter A, Tang P, Reutzel-Selke A, Andreou A, et al. Procedure for decellularization of rat livers in an oscillating-pressure perfusion device. J Visual Exp. 2015:e53029.
Elebring E, Kuna VK, Kvarnström N, Sumitran-Holgersson S. Cold-perfusion decellularization of whole-organ porcine pancreas supports human fetal pancreatic cell attachment and expression of endocrine and exocrine markers. J Tissue Eng. 2017;8:2041731417738145.
Park SM, Yang S, Rye S-M, Choi SW. Effect of pulsatile flow perfusion on decellularization. BioMed Central. 2018;17:1–10.
Say S, Dugos N, Roces S, Mondragon JM. Effect of sonication power on perfusion decellularization of cadaveric porcine kidney. MATEC Web of Conferences. 2019;268:01009.
Manalastas TM, Dugos N, Ramos G, Mondragon JM. Effect of decellularization parameters on the efficient production of kidney bioscaffolds. Appl Biochem Biotechnol. 2020. https://doi.org/10.1007/s12010-020-03338-2.
Schilling BK, Lamberti KK, Snowden MJ, Baker JS, Byrd K, Komatsu C, et al. Design and fabrication of an automatable, 3D printed perfusion device for tissue infusion and perfusion engineering. Tissue Eng Part A. 2020;26:253–64.
Willemse J, Verstegen MMA, Vermeulen A, Schurink IJ, Roest HP, Laan LJW, et al. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mat Sci Eng C. 2020;108:110200.
Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–42.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83.
Mirmalek-Sani SH, Sullivan DC, Zimmerman C, Shupe TD, Petersen BE. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am J Pathol. 2013;183:558–65.
Ji R, Zhang N, You N, Li Q, Liu W, Jiang N, et al. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials. 2012;33:8995–9008.
Sabetkish S, Kajbafzadeh AM, Sabetkish N, Khorramirouz R, Akbarzadeh A, Seyedian SL, et al. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix liver scaffolds. J Biomed Mater Res A. 2015;103:1498–508.
Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell–ECM interactions to tissue engineering. J Cell Physiol. 2004;199:174–80.
Li Q, Uygun BE, Geerts S, Ozer S, Scalf M, Gilpin SE, et al. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials. 2016;75:37–46.
Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, Al-Akkad W, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015;5:13079.
Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21.
Mattei G, Di Patria V, Tirella A, Alaimo A, Elia G, Corti A, et al. Mechanostructure and composition of highly reproducible decellularized liver matrices. Acta Biomater. 2014;10:875–82.
Everwien H, Ariza de Schellenberger A, Haep N, Tzschätzsch H, Pratschke J, Sauer IM, et al. Magnetic resonance elastography quantification of the solid-to-fluid transition of liver tissue due to decellularization. J Mech Behav Biomed Mater. 2020;104:103640.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322.
Rossano JW, Cabrera AG, Jefferies JL, Naim MP, Humlicek T. Pediatric cardiac Intensive Care Society 2014 consensus statement: pharmacotherapies in cardiac critical care chronic heart failure. Pediat Crit Care Med. 2016;17:S20–34.
Tonsho M, Michel S, Ahmed Z, Alessandrini A, Madsen JC. Heart transplantation: challenges facing the field. Cold Spring Harb Perspect Med. 2014;4:a015636.
Harken DE, Soroff HS, Taylor WJ, Lefemine AA, Gupta SK, Lunzer S. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg. 1960;40:744–62.
Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–5.
Ross DN. Replacement of aortic and mitral valves with A pulmonary autograft. Lancet. 1967;2:956–8.
Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525–32.
Cebotari S, Lichtenberg A, Tudorache I, Hilfiker A, Mertsching H, Leyh R,et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation. 2006;114:I132–7.
Cebotari S, Tudorache I, Ciubotaru A, Boethig D, Sarikouch S, Goerler A, et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation. 2011;124:S115–23.
Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–44.
Wong ML, Griffiths LG. Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization. Acta Biomater. 2014;10:1806–16.
Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065–74.
Zhou J, Fritze O, Schleicher M, Wendel HP, Schenke-Layland K, Harasztosi C, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31:2549–54.
Weymann A, Loganathan S, Takahashi H, Schies C, Claus B, Hirschberg K, et al. Development and evaluation of a perfusion decellularization porcine heart model–generation of 3-dimensional myocardial neoscaffolds. Circul J. 2011;4:852–60.
Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, et al. The quest for an optimized protocol for whole-heart decellularization. A comparison of three popular and a novel Decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods. 2011;17:915–26.
Remlinger NT, Wearden PD, Gilbert TW. Procedure for decellularization of porcine heart by retrograde coronary perfusion. J Visual Exp. 2012;70:e50059-e.
Weymann A, Patil NP, Sabashnikov A, Jungebluth P, Korkmaz S, Li S, et al. Bioartificial heart: a human-sized porcine model--the way ahead. PLoS One. 2014;9:e111591.
Oberwallner B, Brodarac A, Choi YH, Saric T, Anić P, Morawietz L, et al. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. J Biomed Mater Res A. 2014;102:3263–72.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43.
Dong J, Li Y, Mo X. The study of a new detergent (octyl-glucopyranoside) for decellularizing porcine pericardium as tissue engineering scaffold. J Surg Res. 2013;183:56–67.
Cicha I, Rüffer A, Cesnjevar R, Glöckler M, Agaimy A, Daniel WG, et al. Early obstruction of decellularized xenogenic valves in pediatric patients: involvement of inflammatory and fibroproliferative processes. Cardiovasc Pathol. 1900;20:222–31.
Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013;2:57–71.
Wong ML, Leach JK, Athanasiou KA, Griffiths LG. The role of protein solubilization in antigen removal from xenogeneic tissue for heart valve tissue engineering. Biomaterials. 2011;32:8129–38.
Kawasaki T, Kirita Y, Kami D, Kitani T, Ozaki C, Itakura Y, et al. Novel detergent for whole organ tissue engineering. J Biomed Mater Res A. 2015;103:3364–73.
Hülsmann J, Aubin H, Bandesha ST, Kranz A, Stoldt VR, Lichtenberg A, et al. Rheology of perfusates and fluid dynamical effects during whole organ decellularization: a perspective to individualize decellularization protocols for single organs. Biofabrication. 2015;7:035008.
Lee PF, Chau E, Cabello R, Yeh AT, Sampaio LC, Gobin AS, et al. Inverted orientation improves decellularization of whole porcine hearts. Acta Biomater. 2017;49:181–91.
Garcia O, Carraro G, Navarro S, Bertoncello I, McQualter J, Driscoll B, et al. Cell-based therapies for lung disease. Br Med Bull. 2012;101:147–61.
Guler S, Aydin HM, Lü LX, Yang Y. Improvement of decellularization efficiency of porcine aorta using dimethyl sulfoxide as a penetration enhancer. Artif Organs. 2018;42:219–30.
Peralta M, Steed E, Harlepp S, González-Rosa JM, Monduc F, Ariza-Cosano A, et al. Heartbeat-driven pericardiac fluid forces contribute to epicardium morphogenesis. Curr Biol. 2013;23:1726–35.
Garanich JS, Mathura RA, Shi Z-D, Tarbell JM. Effects of fluid shear stress on adventitial fibroblast migration: implications for flow-mediated mechanisms of arterialization and intimal hyperplasia. Am J Phys Heart Circ Phys. 2007;292:H3128–35.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233-43.
Pereira RHA, Prado AR, Caro LFCD, Zanardo TÉC, Alencar AP, Nogueira BV. A non-linear mathematical model using optical sensor to predict heart decellularization efficacy. Sci Rep. 2019;9:12211.
Reddy N, Reddy R, Jiang Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015;33:362–9.
Assmann A, Delfs C, Munakata H, Schiffer F, Horstkötter K, Huynh K, et al. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials. 2013;34:6015–26.
Lal MK, Manktelow BN, Draper ES, Field DJ. Chronic lung disease of prematurity and intrauterine growth retardation: a population-based study. Pediatrics. 2003;111:483–7.
Prakash YS, Tschumperlin DJ, Stenmark KR. Coming to terms with tissue engineering and regenerative medicine in the lung. Am J Physiol Lung Cell Mol Physiol. 2015;309:L625–38.
Hardy JD, Webb WR, Dalton ML Jr, Walker GR Jr. Lung homotransplantation in man: report of the initial case. JAMA. 1963;186:1065–74.
Pinsker KL, Koerner SK, Kamholz SL, Hagstrom JW, Veith FJ. Effect of donor bronchial length on healing: a canine model to evaluate bronchial anastomotic problems in lung transplantation. J Thorac Cardiovasc Surg. 1979;77:669–73.
Hayama M, Date H, Oto T, Aoe M, Andou A, Shimizu N. Improved lung function by means of retrograde flush in canine lung transplantation with non-heart-beating donors. J Thorac Cardiovasc Surg. 2003;125:901–6.
Veith FJ, Kamholz SL, Mollenkopf FP, Montefusco CM. Lung transplantation 1983. Transplantation. 1983;35:271–8.
Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb SB, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-second official adult lung and heart-lung transplantation report-2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34:1264–77.
Mallory GB, Spray TL. Paediatric lung transplantation. Eur Respir J. 2004;24:839–45.
Khan MS, Heinle JS, Adachi I, Shirkey B, Schecter MG, Mallory GB, et al. Is lung transplantation survival better in infants? Analysis of over 80 infants. J Heart Lung Transplant. 2013;32:44–9.
Shigemura N, Hattler B, Bermudez C, McCurry KR, Toyoda Y. Impact of graft volume reduction for oversized grafts on outcome after lung transplantation in recipients with end-stage restrictive pulmonary diseases. J Heart Lung Transplant. 2009;28:130–4.
Shigemura N, D'Cunha J, Bhama JK, Shiose A, Abou El Ela A, Hackmann A, et al. Lobar lung transplantation: a relevant surgical option in the current era of lung allocation score. Ann Thorac Surg. 2013;96:451–6.
Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33.
Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41.
Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–52.
Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A. 2013;19:2045–62.
O’Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96:1046–56.
Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700.
Murray JC, Liotta L, Rennard SI, Martin GR. Adhesion characteristics of murine metastatic and nonmetastatic tumor cells in vitro. Cancer Res. 1980;40:347–51.
Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995;108:1251.
Balestrini JL, Gard AL, Gerhold KA, Wilcox EC, Liu A, Schwan J, et al. Comparative biology of decellularized lung matrix: implications of species mismatch in regenerative medicine. Biomaterials. 2016;102:220–30.
Bonenfant NR, Sokocevic D, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials. 2013;34:3231–45.
Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35:3281–97.
Scarritt ME, Bonvillain RW, Burkett BJ, Wang G, Glotser EY, Zhang Q, et al. Hypertensive rat lungs retain hallmarks of vascular disease upon decellularization but support the growth of mesenchymal stem cells. Tissue Eng Part A. 2014;20:1426–43.
Nichols JE, La Francesca S, Vega SP, Niles JA, Argueta LB, Riddle M, et al. Giving new life to old lungs: methods to produce and assess whole human paediatric bioengineered lungs. J Tissue Eng Regen Med. 2017;11:2136–52.
Stahl EC, Bonvillain RW, Skillen CD, Burger BL, Hara H, Lee W, et al. Evaluation of the host immune response to decellularized lung scaffolds derived from α-gal knockout pigs in a non-human primate model. Biomaterials. 2018;187:93–104.
Reing JE, Brown BN, Daly KA, Freund JM, Gilbert TW, Hsiong SX, et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31:8626–33.
Maghsoudlou P, Georgiades F, Tyraskis A, Totonelli G, Loukogeorgakis SP, Orlando G, et al. Preservation of micro-architecture and angiogenic potential in a pulmonary acellular matrix obtained using intermittent intra-tracheal flow of detergent enzymatic treatment. Biomaterials. 2013;34:6638–48.
Wang Z, Wang Z, Yu Q, Xi H, Weng J, Du X, et al. Comparative study of two perfusion routes with different flow in decellularization to harvest an optimal pulmonary scaffold for recellularization. J Biomed Mater Res A. 2016;104:2567–75.
Uriarte JJ, Nonaka PN, Campillo N, Palma RK, Melo E, de Oliveira LVF, et al. Mechanical properties of acellular mouse lungs after sterilization by gamma irradiation. J Mech Behav Biomed Mater. 2014;40:168–77.
Nonaka PN, Campillo N, Uriarte JJ, Garreta E, Melo E, de Oliveira LVF, et al. Effects of freezing/thawing on the mechanical properties of decellularized lungs. J Biomed Mater Res A. 2014;102:413–9.
Calle EA, Hill RC, Leiby KL, Le AV, Gard AL, Madri JA, et al. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater. 2016;46:91–100.
Linhardt RJ, Toida T. Role of glycosaminoglycans in cellular communication. Acc Chem Res. 2004;37:431–8.
Schultz V, Suflita M, Liu X, Zhang X, Yu Y, Li L, et al. Heparan sulfate domains required for fibroblast growth factor 1 and 2 Signaling through fibroblast growth factor receptor 1c. J Biol Chem. 2017;292:2495–509.
Gilpin SE, Wagner DE. Acellular human lung scaffolds to model lung disease and tissue regeneration. Eur Respir Rev. 2018;27:180021.
da Palma RK, Fratini P, Schiavo Matias GS, Cereta AD, Guimarães LL, Anunciação ARDA, et al. Equine lung decellularization: a potential approach for in vitro modeling the role of the extracellular matrix in asthma. J Tiss Eng. 2018;9:2041731418810164.
Nichols WG, Steel HM, Bonny T, Adkison K, Curtis L, Millard J, et al. Hepatotoxicity observed in clinical trials of aplaviroc (GW873140). Antimicrob Agents Chemother. 2008;52:858–65.
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–71.
Moore FD, Smith LL, Burnap TK, Dallenbach FD, Dammin GJ, Gruber UF, et al. One-stage homotransplantation of the liver following total hepatectomy in dogs. Transplant Bull. 1959;6:103–7.
Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–76.
Hannoun Z, Steichen C, Dianat N, Weber A, Dubart-Kupperschmitt A. The potential of induced pluripotent stem cell derived hepatocytes. J Hepatol. 2016;65:182–99.
Marinho A. A study on organ transplantation waiting lines in Brazil’s unified national health system. Cad Saude Publica. 2006;22:2229–39.
Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A. 2011;108:11842–7.
Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288–98.
Lee CA, Dhawan A, Smith RA, Mitry RR, Fitzpatrick E. Instant blood-mediated inflammatory reaction in hepatocyte transplantation: current status and future perspectives. Cell Transplant. 2016;25:1227–36.
Zhang J, Zhao X, Liang L, Li J, Demirci U, Wang S. A decade of progress in liver regenerative medicine. Biomaterials. 2018;157:161–76.
Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20.
Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis. 2010;6:134–6.
Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223.
De Kock J, Ceelen L, De Spiegelaere W, Casteleyn C, Claes P, Vanhaecke T, et al. Simple and quick method for whole-liver decellularization: a novel in vitro three-dimensional bioengineering tool? Arch Toxicol. 2011;85:607–12.
Ren H, Shi X, Tao L, Xiao J, Han B, Zhang Y, et al. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int. 2013;33:448–58.
Jiang WC, Cheng YH, Yen MH, Chang Y, Yang VW, Lee OK. Cryo-chemical decellularization of the whole liver for mesenchymal stem cells-based functional hepatic tissue engineering. Biomaterials. 2014;35:3607–17.
Mattei G, Di Patria V, Tirella A, Alaimo A, Elia G, Corti A, et al. Mechanostructure and composition of highly reproducible decellularized liver matrices. Acta Biomater. 2014;10:875–82.
Gómez MP, Pérez B, Manyalich M. International registry in organ donation and transplantation—2013. Transplant Proc. 2014;46:1044–8.
Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–31.
Wang Y, Bao J, Wu X, Wu Q, Li Y, Zhou Y, et al. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci Rep. 2016;6:24779.
Struecker B, Butter A, Hillebrandt K, Polenz D, Reutzel-Selke A, Tang P, et al. Improved rat liver decellularization by arterial perfusion under oscillating pressure conditions. J Tissue Eng Regen Med. 2017;11:531–41.
Willemse J, Verstegen MMA, Vermeulen A, Schurink IJ, Roest HP, van der Laan LJW, et al. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater Sci Eng C Mater Biol Appl. 2020;108:110200.
Mani NS, Manoj MG, Malik A. Concurrent renal tuberculosis and renal cell carcinoma: A coincidental finding. Indian J Nephrol. 2013;23:237.
Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int Suppl. 2005;68:S7–10.
Mann BS, Manns BJ, Barnieh L, Oliver MJ, Devoe D, Lorenzetti D, et al. Peritoneal dialysis: a scoping review of strategies to maximize PD utilization. Perit Dial Int. 2017;37:159–64.
Chacko MP, Mathan A, Daniel D, Basu G, Varughese S. Significance of pre-transplant anti-HLA antibodies detected on an ELISA mixed antigen tray platform. Indian J Nephrol. 2013;23:351–3.
Anil Kumar BN, Mattoo SK. Organ transplant & the psychiatrist: an overview. Indian J Med Res. 2015;141:408–16.
Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–51.
Hussein KH, Park KM, Lee YS, Woo JS, Kang BJ, Choi KY, et al. New insights into the pros and cons of cross-linking decellularized bioartificial organs. Int J Artif Organs. 2017;40:136–41.
Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–47.
Caralt M, Uzarski JS, Iacob S, Obergfell KP, Berg N, Bijonowski BM, et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am J Transplant. 2015;15:64–75.
Chani B, Puri V, Sobti RC, Jha V, Puri S. Decellularized scaffold of cryopreserved rat kidney retains its recellularization potential. PLoS One. 2017;12:e0173040.
Feng H, Xu Y, Luo S, Dang H, Liu K, Sun WQ. Evaluation and preservation of vascular architectures in decellularized whole rat kidneys. Cryobiology. 2020;95:72–9.
Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16:2207–16.
Nakayama KH, Lee CC, Batchelder CA, Tarantal AF. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8:e64134.
Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Renal tissue engineering with decellularized rhesus monkey kidneys: age-related differences. Tissue Eng Part A. 2011;17:2891–901.
Hussein KH, Saleh T, Ahmed E, Kwak HH, Park KM, Yang SR, et al. Biocompatibility and hemocompatibility of efficiently decellularized whole porcine kidney for tissue engineering. J Biomed Mater Res A. 2018;106:2034–47.
Poornejad N, Momtahan N, Salehi AS, Scott DR, Fronk CA, Roeder BL, et al. Efficient decellularization of whole porcine kidneys improves reseeded cell behavior. Biomed Mater. 2016;11(2):025003.
Gifford S, Zambon JP, Orlando G. Recycling organs-growing tailor-made replacement kidneys. Regen Med. 2015;10:913–5.
Orlando G, Booth C, Wang Z, Totonelli G, Ross CL, Moran E, et al. Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials. 2013;34:5915–25.
Peloso A, Petrosyan A, Da Sacco S, Booth C, Zambon JP, OʼBrien T, et al. Renal extracellular matrix scaffolds from discarded kidneys maintain glomerular morphometry and vascular resilience and retains critical growth factors. Transplantation. 2015;99:1807–16.
Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:6526-9.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7.
Destefani AC, Sirtoli GM, Nogueira BV. Advances in the Knowledge about Kidney Decellularization and Repopulation. Front Bioeng Biotechnol. 2017;5:34.
Mayorca-Guiliani AE, Madsen CD, Cox TR, Horton ER, Venning FA, Erler JT. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat Med. 2017;23:890–8.
Kajbafzadeh AM, Khorramirouz R, Nabavizadeh B, Ladi Seyedian SS, Akbarzadeh A, Heidari R, et al. Whole organ sheep kidney tissue engineering and in vivo transplantation: effects of perfusion-based decellularization on vascular integrity. Mater Sci Eng C Mater Biol Appl. 2019;98:392–400.
Parmaksiz M, Dogan A, Odabas S, Elçin AE, Elçin YM. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed Mater. 2016;11:022003.
Kadish AH. Automation control of blood sugar. I. A servomechanism for glucose monitoring and control. Am J Med Electron. 1964;3:82–6.
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8.
Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34:6760–72.
Rogers J, Farney AC, Al-Geizawi S, Iskandar SS, Doares W, Gautreaux MD, et al. Pancreas transplantation: lessons learned from a decade of experience at Wake Forest Baptist Medical Center. Rev Diab Stud. 2011;8:17–27.
Yang HK, Yoon KH. Current status of encapsulated islet transplantation. J Diabetes Complicat. 2015;29:737–43.
Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol. 2015;3:43.
Zhang Y, Jalili RB, Warnock GL, Ao Z, Marzban L, Ghahary A. Three-dimensional scaffolds reduce islet amyloid formation and enhance survival and function of cultured human islets. Am J Pathol. 2012;181:1296–305.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83.
De Carlo E, Baiguera S, Conconi MT, Vigolo S, Grandi C, Lora S, et al. Pancreatic acellular matrix supports islet survival and function ina synthetic tubular device: in vitro and in vivo studies. Int J Mol Med. 2010;25:195–202.
Kuna VK, Kvarnström N, Elebring E, Holgersson SS. Isolation and decellularization of a whole porcine pancreas. J Vis Exp. 2018:e58302.
Peloso A, Urbani L, Cravedi P, Katari R, Maghsoudlou P, Fallas ME, et al. The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann Surg. 2016;264:169–79.
Huang YB, Mei J, Yu Y, Ding Y, Xia W, Yue T, et al. Comparative decellularization and recellularization of normal versus streptozotocin-induced diabetes mellitus rat pancreas. Artif Organs. 2019;43:399–412.
Wu D, Wan J, Huang Y, Guo Y, Xu T, Zhu M, et al. 3D culture of MIN-6 cells on decellularized pancreatic scaffold: in vitro and in vivo study. Biomed Res Int. 2015;2015:432645.
Dew L, MacNeil S, Chong CK. Vascularization strategies for tissue engineers. Regen Med. 2015;10:211–24.
Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35.
Hegyi P, Petersen OH. The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2013;165:1–30.
Mikhailova AG, Rumsh LD, Dalgalarrondo M, Chobert JM, Haertle T. Activities of anionic and cationic trypsins in the temperature range from 5 to 37 degrees C. Mutant anionic trypsins as a model of cold-adapted (psychrophilic) enzymes. Biochemistry (Mosc). 2003;68:926–33.
Nichols SP, Koh A, Brown NL, Rose MB, Sun B, Slomberg DL, et al. The effect of nitric oxide surface flux on the foreign body response to subcutaneous implants. Biomaterials. 2012;33:6305–12.
Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6.
Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1687–94.
Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–91.
Matsumoto T, Holmes RH, Burdick CO, Metzger JF, Heisterkamp CA 3rd, O'Connell TJ Jr. A study of inverted intestinal graft in the major veins. Angiology. 1966;17:842–50.
Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33:3401–10.
Maghsoudlou P, Totonelli G, Loukogeorgakis SP, Eaton S, De Coppi P. A decellularization methodology for the production of a natural acellular intestinal matrix. J Vis Exp. 2013:e50658.
Patil PB, Chougule PB, Kumar VK, Almström S, Bäckdahl H, Banerjee D, et al. Recellularization of acellular human small intestine using bone marrow stem cells. Stem Cells Transl Med. 2013;2:307–15.
Nowocin AK, Southgate A, Gabe SM, Ansari T. Biocompatibility and potential of decellularized porcine small intestine to support cellular attachment and growth. J Tissue Eng Regen Med. 2016;10:E23–33.
Dew L, English WR, Chong CK, MacNeil S. Investigating neovascularization in rat decellularized intestine: an in vitro platform for studying angiogenesis. Tissue Eng Part A. 2016;22:1317–26.
Urbani L, Camilli C, Phylactopoulos DE, Crowley C, Natarajan D, Scottoni F, et al. Multi-stage bioengineering of a layered oesophagus with in vitro expanded muscle and epithelial adult progenitors. Nat Commun. 2018;9:4286.
Piccoli M, D’Angelo E, Crotti S, Sensi F, Urbani L, Maghin E, et al. Decellularized colorectal cancer matrix as bioactive microenvironment for in vitro 3D cancer research. J Cell Physiol. 2018;233:5937–48.
Kajbafzadeh AM, Khorramirouz R, Masoumi A, Keihani S, Nabavizadeh B. Decellularized human fetal intestine as a bioscaffold for regeneration of the rabbit bladder submucosa. J Pediatr Surg. 2018;53:1781–8.
Giuffrida P, Curti M, Al-Akkad W, Biel C, Crowley C, Frenguelli L, et al. Decellularized human gut as a natural 3D platform for research in intestinal fibrosis. Inflamm Bowel Dis. 2019;25:1740–50.
Chen HJ, Shuler ML. Engineering a bioartificial human colon model through decellularization and recellularization. Methods Mol Biol. 2019;1907:91–102.
Gosztyla C, Ladd MR, Werts A, Fulton W, Johnson B, Sodhi C, et al. A comparison of sterilization techniques for production of decellularized intestine in mice. Tissue Eng Part C Methods. 2020;26:67–79.
Cao G, Huang Y, Li K, Fan Y, Xie H, Li X. Small intestinal submucosa: superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J Mater Chem B. 2019;7:5038–55.
Stanton AL, Lobel M, Sears S, DeLuca RS. Psychosocial aspects of selected issues in women’s reproductive health: current status and future directions. J Consult Clin Psychol. 2002;70:751–70.
Hellström M, Bandstein S, Brännström M. Uterine tissue engineering and the future of uterus transplantation. Ann Biomed Eng. 2017;45:1718–30.
Santoso EG, Yoshida K, Hirota Y, Aizawa M, Yoshino O, Kishida A, et al. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS One. 2014;9:e103201.
Hellström M, El-Akouri RR, Sihlbom C, Olsson BM, Lengqvist J, Bäckdahl H, et al. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014;10:5034–42.
Campo H, Baptista PM, López-Pérez N, Faus A, Cervelló I, Simón C. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol Reprod. 2017;96:34–45.
Padma AM, Tiemann TT, Alshaikh AB, Akouri R, Song MJ, Hellström M. Protocols for rat uterus isolation and decellularization: applications for uterus yissue engineering and 3D cell culturing. Methods Mol Biol. 2018;1577:161–75.
Miki F, Maruyama T, Miyazaki K, Takao T, Yoshimasa Y, Katakura S, et al. The orientation of a decellularized uterine scaffold determines the tissue topology and architecture of the regenerated uterus in rats. Biol Reprod. 2019;100:1215–27.
Daryabari SS, Kajbafzadeh AM, Fendereski K, Ghorbani F, Dehnavi M, Rostami M, et al. Correction to: development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J Assist Reprod Genet. 2020;37:491.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Huang, Y., Yue, H., Lian, Z., Li, X. (2021). The Decellularization of Whole Organs. In: Li, X., Xie, H. (eds) Decellularized Materials. Springer, Singapore. https://doi.org/10.1007/978-981-33-6962-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-33-6962-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6961-0
Online ISBN: 978-981-33-6962-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)