Abstract
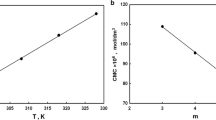
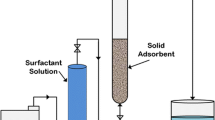
The adsorption of newly synthesized surfactant on Berea core is reported here. The static adsorption surfactant named UTP surfactant was found 0.91 mg/g. The adsorption of the surfactant followed a typical four-region adsorption isotherm. The critical micelle concentration of surfactant was found 2,075 ppm, and the point of zero charge (PZC) of Berea sandstone determined by potentiometric mass titration method (PMT) was at pH 8. The comparison of the adsorption of surfactant with commercial alpha olefin sulfonate (AOS) surfactant was also studied and reported. The newly developed surfactant performed better than AOS. The adsorption behaviors of both surfactants are explained by considering the factors such as the difference in structure, chain length, purity, and critical micelle concentration (CMC).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Q. Feng, et al., “Adsorption of lead and mercury by rice husk ash,” Journal of Colloid and Interface Science, vol. 278, pp. 1-8, 2004.
R. J. Mazen Ahmed Muherei “Equilibrium Adsorption Isotherms of Anionic, Nonionic Surfactants and Their Mixtures to Shale and Sandstone,” Modern Applied Science, vol. Vol. 3, No. 2, 2009.
A. Mohamed, et al., “Hybrid CO2-philic Surfactants with Low Fluorine Content,” Langmuir, vol. 28, pp. 6299-6306, 2012/04/17 2012.
J. Eastoe, et al., “Design and Performance of Surfactants for Carbon Dioxide,” in Supercritical Carbon Dioxide. vol. 860, ed: American Chemical Society, 2003, pp. 285-308.
A. V. Yazdi and E. J. Beckman, “Design of Highly CO2-Soluble Chelating Agents. 2. Effect of Chelate Structure and Process Parameters on Extraction Efficiency,” Industrial & Engineering Chemistry Research, vol. 36, pp. 2368-2374, 1997/06/01 1997.
M. Farooq, et al., “Physiochemical Properties of γ-Al2O3–MgO and γ-Al2O3–CeO2 Composite Oxides,” Journal of Chemical Engineering, vol. 57, pp. 26-32, 2012.
P. Somasundaran and L. Zhang, “Adsorption of surfactants on minerals for wettability control in improved oil recovery processes,” Journal of Petroleum Science and Engineering, vol. 52, pp. 198-212, 2006.
S. Paria and K. C. Khilar, “A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface,” Advances in Colloid and Interface Science, vol. 110, pp. 75-95, 2004.
M. Sagir, I.M. Tan, M. Mushtaq, L. Ismail, M. Nadeem, M.R. Azam, et al., “Novel surfactant for the reduction of CO2/Brine Interfacial tension,” Journal of Dispersion Science and Technology, vol. 35, pp. 463–70, 2013.
M. Sagir, I.M. Tan, M. Mushtaq, S.H. Talebian, “FAWAG using CO2 philic surfactants for CO2 mobility control for enhanced oil recovery applications” in SPE saudi arabia section technical symposium and exhibition, Society of Petroleum Engineers, Al-Khobar, Saudi Arabia, 2014.
M.R. Azam, I.M. Tan, L. Ismail, M. Mushtaq, M. Nadeem, M. Sagir, “Kinetics and equilibria of synthesized anionic surfactant onto berea sandstone,” Journal of Dispersion Science and Technology, vol. 35, pp. 223–30, 2013.
M. Mushtaq, I.M. Tan, M. Nadeem, C. Devi, S.Y.C. Lee, M. Sagir, et al., Grasas y Aceites, vol. 64, pp. 103–114 2013.
Acknowledgments
Authors acknowledge the financial support of UTP and PETRONAS Research Sdn. Bhd. (PRSB) through PRF Project 158200042 and the usage of CEOR Centre of excellence facilities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Singapore
About this paper
Cite this paper
Sagir, M., Tan, I.M., Mushtaq, M., Talebian, S.H. (2015). Static Adsorption of New CO2 Philic Surfactant onto Berea Sandstone. In: Awang, M., Negash, B., Md Akhir, N., Lubis, L. (eds) ICIPEG 2014. Springer, Singapore. https://doi.org/10.1007/978-981-287-368-2_12
Download citation
DOI: https://doi.org/10.1007/978-981-287-368-2_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-367-5
Online ISBN: 978-981-287-368-2
eBook Packages: EnergyEnergy (R0)