Abstract
Cesium in soil exists in two forms. The soil in Fukushima Prefecture contaminated with radioactive cesium was classified into two fractions: the fraction of cesium with weak adsorption to soil (F1), and the fraction of cesium with strong adsorption to soil (F2). We evaluated the absorption of Cs by different crops (rice, soybean, and buckwheat) in different soil fractions by measuring the 133Cs and 137Cs concentrations in the soil and crops. We also evaluated the effect of potassium fertilization on cesium suppression in different soil fractions.
Without K fertilization, 137Cs was absorbed mostly from the F1 fraction by all three crops; 133Cs was absorbed mostly from the F2 and F1 fractions by soybean and buckwheat, respectively, and from both F1 and F2 fractions by rice. Potassium fertilization decreased the percentages of both 137Cs and 133Cs absorbed from the F2 fraction by soybean. In contrast, buckwheat presented reduced absorption from the F1 fraction. In rice, the uptake of cesium by fraction did not change with or without potassium fertilization, indicating that different crops absorb cesium from different soil fractions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The Great East Japan Earthquake that occurred in March 2011 triggered a tsunami that caused extensive damage. In addition to the damage caused by the tsunami, a nuclear accident occurred as a result of an accident at the Tokyo Electric Power Company’s Fukushima Daiichi Nuclear Power Station (hereinafter referred to as “the nuclear accident”). To ensure safe agriculture in this region in the future, the form of radioactive cesium with a long half-life (half-life of 30.2 years, 137Cs) that exists in the agricultural environment and how it is transferred to crops need to be elucidated.
Cesium in soil exists in two forms. One form is electrostatically adsorbed to the surface of mineral particles and can be easily dissolved, while the other form enters adsorption sites such as deep clay layers, binds stably to soil, and cannot be easily dissolved. The radioactive cesium that was released because of the nuclear accident gradually shifted from an easily soluble form to a less soluble form, reaching equilibrium at a certain rate (Fan et al. 2014).
The transfer factor (TF), which is the transfer ratio of radioactive cesium from soil to crops, is often used to evaluate the absorption of cesium (Cs) in soil by crops. However, currently used evaluation methods cannot determine the adsorption of Cs from different soil fractions and cannot accurately evaluate the Cs absorption characteristics of different crop species.
In addition to the radioactive cesium (137Cs) released from the Fukushima Daiichi Nuclear Power Plant, stable isotopes of cesium (133Cs) exist in nature. Both 137Cs and 133Cs behave in the same manner, but the fractions present in the soil differ. In this study, we evaluated the absorption of Cs by different crops in different soil fractions by measuring the 133Cs and 137Cs concentrations in the soil and crops. We also evaluated the effect of potassium fertilization on cesium suppression in different soil fractions.
7.2 Cesium in Each Fraction of Soil
The soil in Fukushima Prefecture contaminated with radioactive cesium was classified into two fractions: the fraction of cesium with weak adsorption to soil, extracted using 1 M ammonium acetate and hydrogen peroxide (F1), and the fraction of cesium with strong adsorption to soil (F2), which was obtained by subtracting F1 from the cesium completely decomposed using hydrofluoric acid. 137Cs in the cultivated crops and in the classified soil was measured using a germanium semiconductor detector and 133Cs was evaluated using ICP-MS.
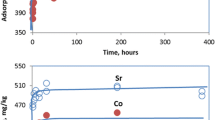
137Cs abundances in the F1 and F2 fractions were approximately 2000 and 17,000 Bq/kg, 10.4% and 89.6% of the total 137Cs, respectively. 133Cs abundances in the F1 and F2 fractions were approximately 130 and 2670 μg/kg, 4.7% and 95.3% of the total 133Cs, respectively. Differences were observed in the fractions of 137Cs and 133Cs. From soil samples collected in 2013, 137Cs, which fell as a result of the nuclear accident, was more abundant than 133Cs in the F1 fraction.
7.3 Intercrop Differences in 137Cs and 133Cs
Soybean, buckwheat, and rice were grown in pots with or without K fertilizer. Figure 7.1 shows the 137Cs and 133Cs contents [μg/kg] for each crop. When no K fertilizer was added, buckwheat absorbed the highest amount of 137Cs per unit weight, whereas soybean and buckwheat absorbed similar amounts of 133Cs per unit weight.
The inhibition of cesium absorption by K fertilization was observed for all crops. Compared with no K fertilization, the percentage reduction in Cs absorption was −31% 137Cs and −52% 133Cs for soybean, −32% 137Cs, and −21% 133C for buckwheat, and −33% 137Cs and −32% 133Cs for rice.
7.4 Transfer Factor for Cs Present in Each Fraction
The Cs absorption from each fraction by the crops was then evaluated. When the transfer factor of Cs from the F1 fraction is TF1 and the soil cesium concentration is C1, and when the transfer factor of Cs from the F2 fraction is TF2 and the soil cesium concentration is C2, the cesium content in the crop (Ccrop) can be expressed as follows:
This equation can be applied to both 133Cs and 137Cs.
Because crops are not considered to absorb 133Cs and 137Cs separately, TF1 and TF2 in Eqs. (7.2) and (7.3) are the same for 133Cs and 137Cs. Therefore, by solving each TF from simultaneous Eqs. (7.2) and (7.3), the following equations are obtained:
Figure 7.2 shows the transition coefficients calculated using Eqs. (7.4) and (7.5).
TF1 tended to be larger in the absence of K fertilization, and buckwheat had the highest TF1 value among the crops. With K fertilization, no significant differences were observed among soybeans, buckwheat, and rice. In contrast, TF2 was less than one-tenth of TF1, being the highest in soybeans in the absence of K fertilization. Conventional transfer factor evaluation could not determine which fraction of the soil absorbed Cs, but this method made it possible to determine the transfer from each fraction of soil.
The amount of cesium absorbed by the crop from each fraction was calculated from the transfer coefficient and the cesium concentration in each fraction. The percentage of Cs in the crop from each fraction is shown in Fig. 7.3. Without K fertilization, 137Cs was absorbed mostly from the F1 fraction by all three crops; 133Cs was absorbed mostly from the F2 and F1 fractions by soybean and buckwheat, respectively, and from both F1 and F2 fractions by rice. Potassium fertilization decreased the percentages of both 137Cs and 133Cs absorbed from the F2 fraction by soybean. In contrast, buckwheat presented reduced absorption from the F1 fraction. In rice, the uptake of cesium by fraction did not change with or without potassium fertilization, indicating that different crops absorb cesium from different soil fractions.
7.5 K Fertilization Suppresses the Absorption of Each Cs Fraction
Furthermore, the difference in cesium concentration in the crop after K fertilization (CcropK) was obtained by multiplying the TF from each fraction absorbed by the crop by the transfer inhibition factor k. The inhibition factor for fraction F1 (soil concentration is C1′) is k1, and that for fraction F2 (soil concentration is C2′) is k2.
Therefore, the inhibition factors k1 and k2 from each fraction could be obtained by simultaneously solving Eqs. (7.8) and (7.9).
It was determined that different crops absorb Cs differently from different soil fractions. The cesium absorbed by soybean without K fertilization was higher than that by buckwheat and rice, indicating that soybean could absorb more cesium that was strongly adsorbed to the soil. In soybean, k1 > k2, and the suppression of the transfer coefficient to the crop by K fertilization had a significant effect on the F2 fraction (Fig. 7.4). Thus, the results suggest that soybean absorbs a greater proportion of K from the F2 fraction in fields with a low K content. Buckwheat had a higher percentage and amount of Cs absorbed from the F1 fraction than soybean and rice, and actively absorbed Cs that was weakly adsorbed in the soil. K fertilization suppressed Cs absorption from the F1 fraction by buckwheat. Because buckwheat has k1 < k2, the Cs suppression effect of K fertilization also affected the absorption from the F1 fraction. Rice absorbed less Cs per unit weight than soybeans and buckwheat. Rice had k1 = k2, with no difference in absorbed fractions observed upon K fertilization in the cultivation test with this test soil, and Cs absorption was similar for both F1 and F2 fractions.
The sequential extraction results for the soils used in this study showed that the extraction rate of 137Cs from the F1 fraction was higher than that of 133Cs, indicating that the soil conditions facilitated the utilization of 137Cs by crops. The difference in Cs uptake by each crop depends on which fraction of Cs is used among the various forms of Cs in the soil. This method that could calculate TF1 and TF2 could identify which fraction of Cs in the crop was derived from each fraction of soil. The results indicate that although the Cs in the crops tested in this study was mainly absorbed from the F1 fraction, most of the Cs was present in the F2 fraction of the soil, which was highly immobilized, and thus contributed a large proportion to the Cs absorption, even if TF2 was small. The extent of absorption from the strongly immobilized F2 fraction of the soil was also determined for each crop. We also determined the suppression of Cs absorption by K fertilization. It has been shown that the transfer coefficient differs among crops, and that it is higher for soybean than for rice as a result of analysis of data after the nuclear power plant accident (IAEA 2020). We hope to clarify the differences in transfer coefficients among crops in the future by utilizing the method presented in this study.
However, this method could not determine the mechanism of absorption from the F2 fraction. It is necessary to clarify through future research whether crops directly absorb Cs from the F2 fraction; whether an equilibrium reaction occurs for Cs, transferring it from the F2 fraction because of a decrease in concentration in the F1 fraction, or a dissolution reaction induced by root-derived organic acids; and the relationship between this indicator and the F2 fraction. The relationship between the Cs concentration in the F1 fraction and this indicator should also be discussed.
References
Fan Q, Yamaguchi N, Tanaka M, Tsukada H, Takahashi Y (2014) Relationship between the adsorption species of cesium and radiocesium interception potential in soils and minerals: an EXAFS study. J Environ Radioact 138:92–100
IAEA (2020) Environmental transfer of radionuclides in Japan following the accident at the Fukushima Daiichi Nuclear Power Plant, TECDOC-1927. IAEA, Vienna
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Furukawa, M., Yoshida, S., Nihei, N. (2023). Evaluation of the Absorption of Different Forms of Cesium from Soil. In: Nakanishi, T.M., Tanoi, K. (eds) Agricultural Implications of Fukushima Nuclear Accident (IV). Springer, Singapore. https://doi.org/10.1007/978-981-19-9361-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-9361-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9360-2
Online ISBN: 978-981-19-9361-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)