Abstract
Radioactive cesium-bearing microparticles (CsMPs) are glassy particles containing large amounts of radioactive cesium (Cs, i.e., 134Cs and 137Cs). Because Cs in CsMPs is covered with insoluble glass, CsMPs may not release Cs into the liquid phase of river water. Previous studies have shown that CsMPs may drive overestimation of Cs transfer between the solid and liquid phases in rivers. In this study, we investigated the contribution of CsMPs to Cs concentrations in forest soil and river water in the Takase River watershed to explore the migration of CsMPs from a forest catchment and their effect on the distribution coefficient in the river water. The Cs concentration derived from CsMPs as a proportion of that in the bulk soil and particulate Cs in the river water was not large; therefore, CsMPs did not have a significant effect on the distribution coefficient. In forest soil, variation in the distribution of CsMPs in soil was greater than that in the distribution of Cs adsorbed onto soil particle. This variation might cause the Cs concentration derived from CsMPs flowing into rivers to vary more than the particulate Cs concentration. To elucidate CsMPs migration and its effects on the Cs concentration in the river, further research such as soil sampling to assess the spatial distribution of CsMPs in the watershed is needed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
19.1 Introduction
Large amounts of radioactive materials were released due to the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident on March 11, 2011 (Chino et al. 2011). In particular, radioactive cesium-137 (137Cs) has a long half-life time of approximately 30 years, and thus, its effect on the environment continues for a long time in contaminated areas (Spezzano 2005). Because radioactive Cs (134Cs and 137Cs) is adsorbed onto clay minerals such as illite (Comans et al. 1991), a large amount of Cs remains in the surface soil layer (Koarashi et al. 2016; Takahashi et al. 2018). Therefore, decontamination activities such as the removal of vegetation and surface soil have been undertaken in residential and agricultural areas since the accident (Evrard et al. 2020). On the other hand, as intensive decontamination activities have not been conducted in forest areas, which occupy approximately 70% of the area of Fukushima Prefecture, large amounts of radioactive materials most likely remain in forest soils (Takahashi et al. 2018; Yoschenko et al. 2022). Cs in the surface soil layer may enter the river due to soil erosion during rainfall (Evrard et al. 2015; Osawa et al. 2018; Niida et al. 2022). In other words, forests may be a source of Cs contamination in downstream areas. Residents have begun to return to heavily impacted areas such as Katsurao Village in 2022 and clarifying the subsequent remigration of radioactive materials from forests to these areas is essential.
Cs-bearing microparticles (CsMPs) were measured in soil and water samples collected in Fukushima Prefecture (Miura et al. 2018; Igarashi et al. 2019; Ikehara et al. 2020). CsMPs are insoluble glassy particles derived from the FDNPP (Adachi et al. 2013; Miura et al. 2018; Ikehara et al. 2020). They have 137Cs concentration per unit mass more than 100,000 times higher than clay minerals that adsorbed 137Cs (Igarashi et al. 2019). Because the particle size of CsMPs can be as small as a few micrometers, concern has been raised about the effects of internal exposure when CsMP is taken into the body of a living organism. Furthermore, as the Cs in CsMPs is contained in insoluble glassy particles, its release into the liquid phase of river water is more difficult than Cs adsorbed onto clay minerals (Miura et al. 2018; Okumura et al. 2020). The distribution coefficient (Kd) in rivers represents solute transition between the solid and liquid phases. Previous studies have suggested that when large amounts of particles that do not contribute to the Cs transition between the solid phase and the liquid phase are present, such as Cs in CsMPs, the apparent Kd may be higher than the net Kd associated with Cs transition (Konoplev et al. 2016; Miura et al. 2018). As Cs in the liquid phase (i.e., dissolved Cs) corresponds to bioavailable Cs, accurate evaluation of Kd is essential to understand the bioavailability of Cs to plants in surrounding ecosystems (Staunton 1994; Koarashi et al. 2016; Miura et al. 2021). On the other hand, the distribution of CsMPs in the forested watershed and the contributions of CsMPs to Kd and Cs concentrations in soils and river water remain unclear (Miura et al. 2021). Ikehara et al. (2020) investigated the distribution of CsMPs in soils of Fukushima Prefecture. They found a large amount of CsMPs in the soil near the FDNPP. However, the distribution of CsMP in their study was assessed immediately after the accident, and it is now necessary to determine the current distribution of CsMP 11 years after the accident. Furthermore, previous studies have not clarified the distribution of CsMPs in forest soil and its vertical distribution in soil to depths of several tens of centimeters. Assessing the migration of CsMPs from the forest to downstream areas and the effects of CsMP on river water requires comprehensive elucidation of the distribution of CsMPs in forest soils, which provide the source of CsMPs to the river, as well as the inflow of CsMPs into rivers.
In this study, we investigated the distribution of CsMPs in forest soil and the inflow of CsMPs to a river in a forested watershed in Fukushima Prefecture. We evaluated Cs derived from CsMPs as a proportion of the Cs concentrations in forest soil and river water.
19.2 Methods
19.2.1 Study Sites
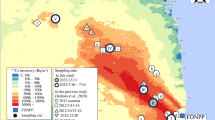
We collected soil and river water samples from the Takase River watershed in Namie Town, Fukushima Prefecture, Japan. The study catchment (37°22′17.2″N to 37°32′37.2″N, 140°40′53.2″E to 140°56′44.4″E) has an area of 244 km2 and the average Cs inventory (i.e., 137Cs + 134Cs) in the surface soil was 980 kBq m−2 in September 2017 (Fig. 19.1) (MEXT 2014). The collection sites of soil and water samples are located 10.8 km and 9.18 km northwest of the FDNPP, respectively.
Sampling sites for forest soils and river water in the Takase River watershed. 137Cs inventory was calculated using data from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT 2014)
19.2.2 Sampling and Analysis of Forest Soils
We collected soil samples from the sampling sites on September 5, 2021. Three soil column samples with soil depths of 0–20 cm were collected at 3 m intervals. After each soil sample was returned to the laboratory, the following treatment was conducted. The soil column samples were sliced into layers from the surface at intervals of one to several centimeters, and the soil sample from each depth was dried in an oven at 100 °C for 24 h. After drying, each sample was passed through a sieve with a pore size of 2.00 mm. The soil that passed through the sieve was placed into a 100 mL U8 container (Pla tsubo 3-20, Umano Kagaku Youki Co., Ltd., Osaka, Japan). The Cs concentration (sum of 134Cs and 137Cs) of the bulk soil sample was measured using a germanium semiconductor detector (GC4020, Canberra Industries Inc., USA).
After measurement of the Cs concentration, a portion of the soil sample (approximately 2–3 g dry soil) was placed into a zip-closure bag and Cs radioactivity derived from CsMPs was measured using an imaging plate (IP; BAS IP MS 2025, Cytiva, Japan) with an IP reader (FLA-9500, GE Healthcare Bio-Science AB, Sweden) (Sagawa et al. 2011; Miura et al. 2018; Ikehara et al. 2018) (Fig. 19.2). The IP can store energy emitted from radioactive materials and be read with an IP reader to create a luminescent image proportional to the radioactive energy. Cs radioactivity at each spot with high energy in the image was calculated using image processing software (ImageJ, National Institutes of Health, USA). In this study, spots with activity of 0.01 Bq or more were assumed to indicate Cs derived from CsMPs. Furthermore, we calculated the proportion of Cs concentration derived from CsMPs to the Cs concentration in the bulk soil at each soil depth, hereinafter referred to as the proportion of CsMPs in the soil with reference to the previous study (Ikehara et al. 2020).
19.2.3 Sampling and Analysis of River Water
River water samples were collected from the Takase River during rainfall events on June 29, July 7, and July 27, 2021, using an automatic programmable water sampler (6712, Teledyne ISCO, USA). Approximately 1.00 L river water was collected over a total of 8 h at 20 min intervals during one rainfall event (i.e., approximately 24 L sample was collected during one rainfall event).
The water samples were filtered in 1 L volumes using a membrane filter with a pore size of 0.45 μm (JHWP004700, Merck Co., Germany) and then divided into suspended solids (SS) and filtrate fractions. After drying the SS on the membrane filter in an oven at 100 °C for 24 h, it was placed in an U8 container, and its Cs concentration was measured using a germanium semiconductor detector. Cs in SS is referred to as particulate Cs. Next, Cs radioactivity derived from CsMP in the SS was measured using an IP as for the soil sample (Fig. 19.3). We determined the proportion of Cs concentration derived from CsMPs to the particulate Cs concentration for each SS sample, hereinafter called the proportion of CsMPs in particulate Cs.
For analysis of the filtrate, the whole sample from one rainfall event (i.e., approximately 24 L) was passed through a single cartridge filter (CS-13ZN, Japan Vilene Co., Japan), and the Cs concentration was measured. Cs in the filtrate is referred to as dissolved Cs. Then, we calculated Kd (i.e., apparent Kd) and Kd without CsMPs (i.e., net Kd) for each sample, respectively (Miura et al. 2018). The Kd was obtained by dividing particulate Cs concentration per unit mass of SS by dissolved Cs concentration in the river water. The Kd without CsMPs was calculated by calculated by subtracting the Cs concentration derived from CsMPs from the suspended Cs concentration and dividing it by the dissolved Cs concentration.
19.3 Results and Discussion
19.3.1 Vertical Distribution of CsMPs in Forest Soil
Figure 19.4 shows the vertical distribution of Cs concentration in each soil layer and in CsMPs, along with the proportion of CsMPs in the soil for each layer. CsMPs were detected in soil samples collected to a depth of 20 cm. This result indicated that CsMPs moved within the soil. Because Cs in the CsMPs is surrounded by an insoluble glassy particle (Adachi et al. 2013), the Cs may not react directly with soil. The Cs in CsMPs may not be fixed to the FES and therefore can move to deeper soil layers. The average proportion of CsMPs in the soil was less than 3% in all soil layers (Fig. 19.4). This result is consistent with previous studies demonstrating that the proportion of CsMPs is small, because many forms of Cs other than CsMPs were distributed near the nuclear power plant (Ikehara et al. 2020). Furthermore, as observed for Cs concentration in the soil, the Cs concentration derived from CsMPs and the proportion of CsMPs decreased with increasing soil depth. No significant difference in the proportion of CsMPs in the soil was found between the layer at 1–10 cm and the surface soil layer at soil depth of 0–1 cm. On the other hand, the proportion of CsMPs in the soil significantly decreased below the soil depth of 10 cm (p < 0.05). These results suggest that CsMPs may migrate more slowly than ionic Cs in soil solution in the soil deeper layer. Few studies have investigated the vertical distribution and dynamics of CsMPs in soils. To elucidate the dynamics of CsMPs, further studies such as soil sampling and analysis of soil physics are needed in the future.
Figure 19.5 shows the variation in Cs concentration in the soil and Cs derived from CsMPs in each soil layer calculated from three soil column samples. In all layers, the Cs concentration derived from CsMPs had a greater coefficient of variation than Cs in the soil. This suggests that the distribution of CsMPs in soil might be more heterogeneous than the distribution of Cs adsorbed onto soil, and that these distributions might not always match.
19.3.2 Discharge of CsMPs from the Forested Catchment
Figure 19.6 shows changes in the particulate Cs concentration and Cs concentration derived from CsMPs for each sample. Figure 19.7 shows relationships of SS concentrations with particulate Cs concentrations and Cs concentrations derived from CsMPs. Particulate Cs was detected in all water samples (Fig. 19.6), and its concentration was positively correlated with SS concentrations (correlation coefficient, r = 0.398). This is consistent with previous studies showing that the discharge of particulate Cs increases with sediment runoff via soil erosion (Evrard et al. 2015; Osawa et al. 2018; Niida et al. 2022). On the other hand, CsMPs were not detected in some water samples (Fig. 19.6). Furthermore, no correlation was found between the Cs concentrations derived from CsMPs and SS concentrations (r = 0.070 in Fig. 19.7b). As noted in Sect. 19.3.1, Cs derived from CsMPs may be more unevenly distributed in the soil than Cs adsorbed onto the soil (Fig. 19.5). Therefore, the influx of CsMPs into rivers due to soil erosion may be less stable than the input of particulate Cs. However, soil sampling in this study was conducted at only one site in the watershed, and therefore, the tendency of CsMPs in the entire watershed cannot be addressed. Determining the distribution of CsMPs over a wide area, such as with a Cs inventory converted from air dose rates by aircraft monitoring (MEXT 2014), is technically difficult. Therefore, clarifying the relationship between the inflow of CsMPs into rivers and the spatial distribution of CsMPs in future research will require surveys in relatively narrow watersheds.
Table 19.1 shows the average Cs concentration by form (i.e., particulate Cs, dissolved Cs, and CsMPs), the proportion of CsMPs in particulate Cs, and the distribution coefficient for each sample. Particulate Cs accounted for more than 90% of Cs discharge during rainfall, in accordance with previous studies (Osawa et al. 2018; Niida et al. 2022). On the other hand, the average proportion of CsMPs in particulate Cs was only 3.46%, indicating that most of the discharged particulate Cs was in forms other than CsMPs. Furthermore, no significant difference was found between Kd and Kd without CsMPs for any sample (p > 0.05). Thus, CsMPs may not significantly affect the Kd of rivers, because the amount of Cs derived from CsMPs is smaller than the amount of Cs present in other forms.
19.4 Conclusion
CsMPs were detected from forest soils and river water in the Takase River watershed in 2021. The proportion of Cs concentration derived from CsMPs in the forest soils was as small as about 3%; therefore, they did not significantly affect the particulate Cs and distribution coefficients in the river water collected during rainfall. It was suggested that the variation of Cs concentration derived from CsMPs in the forest soil was greater than that in the bulk soil and affects the instability of CsMPs flowing into rivers. To grasp the dynamics of CsMPs via soil erosion, further research is needed on the dynamics of CsMPs in soils before they flow into rivers and their distribution in soil in detail.
References
Adachi K, Kajino M, Zaizen Y, Igarashi Y (2013) Emission of spherical cesium-bearing particles from an early stage of the Fukushima nuclear accident. Sci Rep 3:12–15. https://doi.org/10.1038/srep02554
Chino M, Nakayama H, Nagai H, Terada H, Katata G, Yamazawa H (2011) Preliminary estimation of release amounts of 131 I and 137 Cs accidentally discharged from the Fukushima Daiichi Nuclear Power Plant into the atmosphere. J Nucl Sci Technol 48:1129–1134. https://doi.org/10.1080/18811248.2011.9711799
Comans RNJ, Hller M, De Preter P (1991) Sorption of Cs on illite nonequilibrium behavior and reversibility. J Phys Act Health 55:433–440. https://doi.org/10.4319/lo.2013.58.2.0489
Evrard O, Laceby JP, Lepage H, Onda Y, Cerdan O, Ayrault S (2015) Radiocesium transfer from hillslopes to the Pacific Ocean after the Fukushima nuclear power plant accident: a review. J Environ Radioact 148:92–110. https://doi.org/10.1016/j.jenvrad.2015.06.018
Evrard O, Laceby JP, Nakao A (2020) Effectiveness of landscape decontamination following the Fukushima nuclear accident: a review
Igarashi Y, Kogure T, Kurihara Y, Miura H, Okumura T, Satou Y, Takahashi Y, Yamaguchi N (2019) A review of Cs-bearing microparticles in the environment emitted by the Fukushima Dai-ichi nuclear power plant accident. J Environ Radioact 205:101–118. https://doi.org/10.1016/j.jenvrad.2019.04.011
Ikehara R, Suetake M, Komiya T, Furuki G, Ochiai A, Yamasaki S, Bower WR, Law GTW, Ohnuki T, Grambow B, Ewing RC, Utsunomiya S (2018) Novel method of quantifying radioactive cesium-rich microparticles (CsMPs) in the environment from the Fukushima Daiichi nuclear power plant. Environ Sci Technol 52:6390–6398. https://doi.org/10.1021/acs.est.7b06693
Ikehara R, Morooka K, Suetake M, Komiya T, Kurihara E, Takehara M, Takami R, Kino C, Horie K, Takehara M, Yamasaki S, Ohnuki T, Law GTW, Bower W, Grambow B, Ewing RC, Utsunomiya S (2020) Abundance and distribution of radioactive cesium-rich microparticles released from the Fukushima Daiichi nuclear power plant into the environment. Chemosphere 241:125019. https://doi.org/10.1016/j.chemosphere.2019.125019
Koarashi J, Nishimura S, Nakanishi T, Atarashi-Andoh M, Takeuchi E, Muto K (2016) Post-deposition early-phase migration and retention behavior of radiocesium in a litter-mineral soil system in a Japanese deciduous forest affected by the Fukushima nuclear accident. Chemosphere 165:335–341. https://doi.org/10.1016/j.chemosphere.2016.09.043
Konoplev A, Golosov V, Laptev G, Nanba K, Onda Y, Takase T, Wakiyama Y, Yoshimura K (2016) Behavior of accidentally released radiocesium in soil-water environment: looking at Fukushima from a Chernobyl perspective. J Environ Radioact 151:568–578. https://doi.org/10.1016/j.jenvrad.2015.06.019
Ministry of Education Culture, Sports and T (2014) Results of deposition of radioactive cesium of the airborne monitoring survey in the areas to which evacuation orders have been issued. https://radioactivity.nsr.go.jp/ja/contents/7000/6749/24/191_258_0301_18.pdf. Accessed 1 May 2022
Miura H, Kurihara Y, Sakaguchi A, Tanaka K, Yamaguchi N, Higaki S, Takahashi Y (2018) Discovery of radiocesium-bearing microparticles in river water and their influence on the solid-water distribution coefficient (Kd) of radiocesium in the Kuchibuto River in Fukushima. Geochem J 52:145–154. https://doi.org/10.2343/geochemj.2.0517
Miura H, Kurihara Y, Takahashi Y (2021) Environmental dynamics of radiocesium-bearing microparticles emitted from the Fukushima Daiichi nuclear power plant accident: transport from rivers to the ocean and impact on the environment. Geochemistry 55:122–131. https://doi.org/10.14934/chikyukagaku.55.122
Niida T, Wakiyama Y, Takata H, Taniguchi K, Kurosawa H, Fujita K, Konoplev A (2022) A comparative study of riverine 137Cs dynamics during high-flow events at three contaminated river catchments in Fukushima. Sci Total Environ 821:153408. https://doi.org/10.1016/j.scitotenv.2022.153408
Okumura T, Yamaguchi N, Kogure T (2020) Distinction between radiocesium (RCs)-bearing microparticles and RCs-sorbing minerals derived from the Fukushima nuclear accident using acid treatment. Chem Lett 49:1294–1297. https://doi.org/10.1246/CL.200374
Osawa K, Nonaka Y, Nishimura T, Tanoi K, Matsui H, Mizogichi M, Tatsuno T (2018) Quantification of dissolved and particulate radiocesium fluxes in two rivers draining the main radioactive pollution plume in Fukushima, Japan (2013–2016). Anthropocene 22:40–50. https://doi.org/10.1016/j.ancene.2018.04.003
Sagawa N, Yamazaki T, Kurosawa S, Izaki K (2011) JAEA-technology application of imaging plate to the radiation protection in the MOX fuel fabrication facility march 2011 Japan Atomic Energy Agency. IAEA, Vienna
Spezzano P (2005) Distribution of pre- and post-Chernobyl radiocaesium with particle size fractions of soils. J Environ Radioact 83:117–127. https://doi.org/10.1016/j.jenvrad.2005.02.002
Staunton S (1994) Adsorption of radiocaesium on various soils: consequences of the effects of soil:solution composition on the distribution coefficient. Eur J Soil Sci 45:409–418. https://doi.org/10.1111/j.1365-2389.1994.tb00526.x
Takahashi J, Onda Y, Hihara D, Tamura K (2018) Six-year monitoring of the vertical distribution of radiocesium in three forest soils after the Fukushima Dai-ichi nuclear power plant accident. J Environ Radioact 192:15. https://doi.org/10.1016/j.jenvrad.2018.06.015
Yoschenko V, Nanba K, Wada T, Johnson TE, Zhang J, Workman D, Nagata H (2022) Late phase radiocesium dynamics in Fukushima forests post deposition. J Environ Radioact 251:106947. https://doi.org/10.1016/j.jenvrad.2022.106947
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science, grant 20H00435, the FY2021 Research Fund of Environmental Radioactivity Research Network (F-21-28), and the academic fund of the Japanese Society of Irrigation, Drainage and Rural Engineering. We also acknowledge support from the Japan Atomic Energy Agency.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/owKY9s.
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Tatsuno, T., Waki, H., Nagasawa, W., Nihei, N., Murakami, M., Ohte, N. (2023). Contribution of Cesium-Bearing Microparticles to Cesium in Soil and River Water of the Takase River Watershed and Their Effect on the Distribution Coefficient. In: Nakanishi, T.M., Tanoi, K. (eds) Agricultural Implications of Fukushima Nuclear Accident (IV). Springer, Singapore. https://doi.org/10.1007/978-981-19-9361-9_19
Download citation
DOI: https://doi.org/10.1007/978-981-19-9361-9_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9360-2
Online ISBN: 978-981-19-9361-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)