Abstract
We collected undisturbed soil column samples (5 cm in diameter and 30 cm in length) using a liner soil sampler on and near the forest floor. The soil was sliced at predetermined intervals, and then the radioactive Cs concentration, soil organic carbon and nitrogen contents were analyzed. Below the litter layer on the forest floor, we observed more significant downward migration of radioactive Cs 2 years after the accident. In contrast, no such large migration happened at the downslope, which has no surface litter layer. Soil organic carbon content and radioactive Cs content are highly correlated, suggesting organic matters produced by decomposition of litter contributed to enhancing the downward migration of radioactive Cs.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Radioactive materials emitted by the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident in March 2011 are still causing environmental issues in northeastern Japan. The main element of concern is cesium (Cs), which was not often studied in soil science. Cesium behaves differently from Na, Ca, and other metal cations commonly noted in soil physics. The range of Cs concentrations in the environment is considerably lower than that of most cations observed in previous studies on soil solute transport. For example, one million becquerels per kg of soil (Bq kg−1 hereafter) is equivalent to 0.3 ppb in mass. The distribution coefficient of Cs in soil under the ppb concentration range is far greater than that under the ppm order (Comans et al. 1991). Notably, the distribution coefficient of Cs is concentration dependent.
The half-life of 137Cs is relatively longer than that of other radioactive materials emitted from the accident; therefore, pollution from 137Cs and countermeasures must occur over a long period. However, because of cost factors, decontamination of radioactive Cs in forests was limited to the perimeter of the forests, and the fate of 137Cs deposited in forests in Fukushima is an ongoing issue for pollution management.
Cs is a monovalent cation with a large ionic radius (0.169 nm), which results in more significant dehydration than other monovalent cations. Dehydrated Cs cations are often found in the ditrigonal siloxane cavity at the surface of clay plates and frayed edge sites between clay layers. This essentially causes irreversible adsorption of Cs onto clay (Dalvaux et al. 2000). In addition to the structural characteristics of clay minerals, dehydrated Cs exhibits thermodynamic ion-exchange characteristics quite different from those of sodium and divalent cations. As a result Cs prefers adsorption to the solid phase (Appelo and Postma 2005).
A greater distribution coefficient in the solid phase causes retardation of Cs transport in soils. After the FDNPP accident, deposited radioactive Cs was selectively detected in near-surface soil, and its downward migration was slow (Takahashi et al. 2018). After the accident, the government focused on the delay in Cs transport and assumed that radioactive Cs mainly stayed near the surface. Large-scale surface soil scraping was performed based on this assumption. However, the preference for adsorption to solids suggests the possibility of colloid-facilitated transport of radioactive Cs in soil.
This study focused on organic colloids produced by the degradation of surface litter that may enhance the migration of Cs in soils. Soil samples were collected from the surface litter layer on the forest floor, and bare land adjacent to it, and the distribution of radioactive Cs was investigated.
14.2 Study Site and Methods
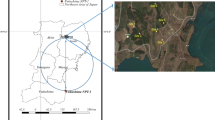
The detailed geomorphology of the Fukushima slope studied is shown in Fig. 14.1. The slope was located approximately 30 km northwest of FDNPP, and had a deposition of radioactive Cs that was delivered by the wind from the southeast on the day of the accident. The region was covered with weathered granite soil.
In May 2013, we collected undisturbed soil column samples (5 cm in diameter and 30 cm in length) using a liner soil sampler (DIK-110C, Daiki Inc., Saitama, Japan). Similar sampling was conducted in the upslope area in 2014.
The samples were taken to the laboratory. Layers of the soil column were photographed, and the soil was cut at predetermined intervals, and then, the radioactive Cs concentration, soil organic carbon content, and soil nitrogen content were analyzed for each depth. For the 20 cm thick surface layer, the soil was cut at 2 cm intervals, with larger intervals for the deeper layer. The sliced samples were oven-dried and ground with a mortar and pestle, and stones and coarse organic matter were removed. The sample was then placed in a 20 mL vial, and the radioactive Cs content was measured using an NaI spectrophotometer (Wizard 2480, Perkin Elmer Inc.). After radioactivity measurement, a sample was taken from the vial, and the organic carbon and nitrogen contents were measured using a CN coder (CN-60, Sumika Inc.).
14.3 Results and Discussion
14.3.1 Surface Conditions and Soil Layers
Photo 14.1 shows the sample obtained in May 2013. Weathered granite soil dominated the area. In general, yellow-weathered granite soil was observed in low organic content subsoil and outcrops of cliffs. The dark color of the soil suggests the presence of organic matter.
Photograph of strata of soil cores sampled in 2013. The number in the photograph denotes the sampling site in Fig. 14.1
In Fig. 14.1, Sites 1, 2, and 8 are soils from the upslope forest floor with a surface litter layer. Neither trace of water erosion nor sediment deposition was observed in the upslope area. Sites 1 and 2 showed a dark color near the surface, which became lighter in the deeper layer. The soil sample from Site 8 showed dark colors from the surface to a depth of 30 cm. Mid-slope areas (Sites 7, 9, 10, and 11) were under complex erosion and sedimentation processes. Some sites experienced erosion, whereas others revealed sediment deposition from the upslope. The mid-slope area was covered with weeds and young tree bush. There was a partial organic matter on the soil surface, but no significant litter layer, such as that observed at the upslope. Site 9 had bird feathers found at a depth of 25 cm, suggesting that most of the soil had been deposited recently. Thus, we omitted soil from Site 9 from further analysis. Soil samples from the mid-slope area showed a dark color near the surface, and yellow soil was strongly affected by the parent material from 13 cm (Sites 10 and 11) to 20 cm (Site 7) in depth. Sediments from the upslope were deposited at the foot of the slope (Sites 3, 4, 5, and 6). Site 3 had black soil in the near-surface layer and the layer deeper than 20 cm. This deeper dark layer suggests it was the former soil surface, and organic matter had accumulated at that time. The dark layer near the surface at Site 3 could be recently deposited sediments from upstream. Site 4 showed different textured loose soils in the 0–5 cm and 5–15 cm layers. This reflects recent sediments from the upslope and may be due to the loose soil structure in the 10 cm deep layer at Site 6. Site 5 showed an accumulation of organic matter and a dark color for the entire layer, from the surface to a depth of 30 cm. Site 5 may have been one of the recessed depositional areas in this slope.
14.3.2 Organic Carbon and Carbon/Nitrogen Ratio
The average organic carbon content and carbon/nitrogen (CN) ratio distributions for each area are shown in Fig. 14.2. The upstream values, UP_2013 and UP_2014, were averages of Sites 1, 2, and 8 in 2013 and 2014, respectively, while midstream (Mid_2013) and downstream (Foot_2013) showed averages for mid-slope areas at Sites 7, 10, and 11, and downstream at 3, 4, 5, and 6, respectively. The upstream area showed a high organic carbon content at 0–8 cm in depth, where a fibrous structure is observed in Photo 14.1. Organic carbon content decreased with increasing depth. The CN ratio of soil from the upslope area decreased slightly with increasing depth. The organic carbon content distribution of the upslope area was similar to that of nonvolcanic ash soil reported for a forest floor in western Japan (Kawahara 1970). No trace of water erosion was observed; therefore, the decomposition of organic materials in surface litter likely supplied secondary organic compounds to the soil layer. Organic compounds migrated to deeper layers with further decay (Kawahara 1970), and this further decay caused a slight decrease in the CN ratio. Secondary organic compounds could be what is generally referred to as dissolved organic carbon in the soil.
The soil sampled at the mid-slope did not show high organic carbon content near the surface, as observed in the upslope area. At approximately 8 cm in depth and deeper, the mid-slope soil showed similar organic carbon levels and CN ratios to the one at the upslope, and both decreased in the deeper layers. The lower organic carbon content for the surface at 0–8 cm layer suggested that removing surface soil by erosion affected the 8 cm thick surface layer, and fresh organic matter with a higher CN ratio was essentially removed. This could have caused low organic carbon in the soil near the surface.
Downstream of the slope, Sites 3, 4, 5, and 6 showed relatively narrow ranges of organic carbon content and CN ratio distributions, possibly due to the deposition of sediments from the upslope.
14.3.3 Radioactive Cs Distributions
Radioactive Cs is rare in nature. The measurement of radiation by an NaI scintillation spectrometer does not give us as precise a measurement as that of a Ge-semiconductor detector. However, dozens or a hundred Bq kg−1 of radioactive Cs detected in the environment using the NaI spectrometer can be traces of a particular event. Soils at the mid-slope and downstream of the slope showed low radioactive Cs in layers deeper than 15 cm, which may indicate the initial soil Cs prior to the FDNPP accident.
The radioactive Cs distribution in the three areas of the slope is shown in Fig. 14.3. Soil samples from the upslope showed high radioactive Cs content for a near-surface layer, with greater than 10,000 Bq kg−1 in layers above 5 cm in depth, and greater than 1000 Bq kg−1 at depths greater than 10 cm. The mid-slope area showed a similar radioactive Cs content distribution as the upslope area for the shallow soil layer (0–10 cm in depth). However, the mid-slope area had a further decrease in radioactive Cs content in the deeper layer, whereas the upslope maintained a greater amount up to 20 cm in depth. Downstream of the slope, a relatively large radioactive Cs content distribution was observed in the shallow layer; however, it was less than 100 Bq kg−1 at depths below 10 cm. Photo 14.1 suggests that downstream of the slope had a loose soil structure at a 10 cm thick surface layer, which could indicate that depositional sediments were the main constituents. The sediments from the upslope area had radioactive Cs, although no further downward migration of the radioactive Cs was observed in the downstream area.
Erosion and sedimentation occurred in the mid-slope area. Photo 14.1 suggests that the 5–10 cm thick surface soils from the mid-slope area were recently deposited sediments, because the structure appeared to be loose. As shown in Fig. 14.3, this layer had a higher radioactive Cs content. Below the sedimentation layer, at a depth greater than 10 cm at Site 10 in Photo 14.1, the radioactive Cs content was distinctly less than that in the upslope area. Additional specific processes must have enhanced the downward migration of radioactive Cs in the soil in the upslope area.
Dissolved Cs move more slowly than water in the soil, and the extent of the delay can be evaluated using the retardation factor (R) as follows:
where Kd is the distribution coefficient (L kg−1), ρd is the dry bulk density, and θ (m3 m−3) is the volumetric water content.
Assuming the distribution coefficient of Cs was 1000 Bq kg−1 as a conservative number (Ishikawa et al. 2007), the dry bulk density of the soil (1.3 g cm−3) and porosity (0.5) produce a retardation factor of 2600 (Appelo and Postma 2005). This suggests that even in a water-saturated condition, radioactive Cs move at a velocity 1/2600 times slower than that of water in the soil. If the soil is unsaturated, the migration of Cs is considerably slower.
Assuming that the annual precipitation of the region is 1370 mm year−1 (Meteorological Agency of Japan) and evapotranspiration is 400 mm year−1 (Kohatake 1989), the average downward water flux density would be 970 mm year−1. The porosity of the soil is 0.5; therefore, the average water flow velocity within the soil would be calculated at 970/0.5 = 1940 mm year−1. Using the retardation factor, the estimated migration velocity of Cs is 1940/2600 = 0.75 mm year−1.
The estimated migration of the dissolved radioactive Cs was 1.5 mm for 2 years, which is less than 1/100 of that observed for the upslope area, with radioactive Cs content near 1000 Bq kg−1 at depths of 20 cm. Therefore, the discussion of dissolved Cs alone cannot explain the large migration of radioactive Cs after the FDNPP accident.
14.3.4 Effects of Surface Litter on the Migration of Radioactive Cs
It was interesting that the soil near the surface above 10 cm in depth at the upslope and mid-slope areas showed high and similar radioactive Cs content distribution, while the upslope area alone still had a high content at those deeper than 10 cm. The organic matter near the surface layer could be a reason for the difference in radioactive Cs distribution between upslope and mid-slope soils.
Figure 14.4 shows the relationship between the radioactive Cs and organic carbon contents of the soil samples. For the upslope area covered by litter with no distinct trace of water erosion, the radioactive Cs content was correlated with soil organic carbon content. The correlation was valid for both soil samples collected in 2013 and 2014, with a high coefficient of determination. Soils from the mid-slope and downstream areas did not show a significant correlation with the organic carbon content (regression is not shown in Fig. 14.4). Complex erosion and deposition processes may have contributed to this result. The mid-slope and downstream regions showed some depositional sediment near the surface. This could disturb the distribution of radioactive Cs in the soil and, thus, their relationship with the organic carbon content.
As mentioned previously, the soil organic carbon and CN ratio distributions of the upslope area were similar to those observed on a forest floor in western Japan under similar conditions of surface litter, nonvolcanic ash soil, and a moist climate. Dissolved and colloidal organic matter can be produced and supplied to the soil by surface organic matter decomposition, which could affect radioactive Cs distribution at the study site. The surface litter had a high amount of radioactive Cs deposited during the FDNPP incident. Decomposition of polluted litter could release radioactive Cs from organic matter, which then migrated into deeper layers in dissolved or colloidal form. In addition, dissolved organic matter produced through surface litter decomposition may also affect the migration of Cs in soil.
Recently, Tatsuno et al. (2022) conducted a column experiment that had different orders of Cs and organic matter load to investigate the role of dissolved organic matter in the migration of radioactive Cs with a concentration found in the natural environment. Those authors compared the migration of Cs in a Cs-dissolved organic matter mixed solution and a Cs solution applied after the addition of a certain amount of dissolved organic matter. They concluded that dissolved organic matter could enhance the migration of radioactive Cs in the soil. This was interpreted as a mask of strong adsorption sites such as the FES by dissolved organic materials, and colloids facilitated the transport of Cs by dissolved organic matter, which enhanced Cs migration. Such mechanisms are expected to play a role at our study site. Concerning Tatsuno et al. (2022), it can be considered that two processes contributed to enhancing the migration of Cs in our study. The long-term supply of dissolved organic matter by the decomposition of surface organic residue and litter that started before the FDNPP accident could have masked the strong adsorption sites of clay minerals such as FES. This could have enhanced the migration of deposited radioactive Cs near the surface soil. After the accident, the decomposition of newly deposited organic materials, which contained radioactive Cs emitted by the accident produced dissolved and colloidal radioactive Cs through the decomposition process. The dissolved Cs showed more downward migration than that reported in previous studies (Yamaguchi et al. 2012), since adsorption sites of the soil near the surface were masked due to the long-term supply of dissolved organic matter. As a result, the upslope and mid-slope areas showed similar radioactive Cs distributions below the litter layer, at approximately 4–10 cm in depth. Decomposition of newly deposited organic matter could also produce colloidal Cs. In addition to Tatsuno et al. (2022), previous studies have reported a decrease in the retardation factor of radioactive elements (Pu, Am) by the addition of colloidal particles into the solution (i.e., Contardi et al. 2001), and an increase in the migration of Cs through the soil (Turner et al. 2006). Organic colloids facilitated Cs transport and were expected to cause the further downward migration of radioactive Cs in the upslope area. As mentioned previously, the concentration ranges of common cations and radioactive Cs are different. One million Bq kg−1 of radioactive Cs is equivalent to 0.3 ppb in mass. When organic colloids facilitated Cs migration, a slightly undetectable amount of organic colloids was effective in altering the radioactive Cs distribution in the soil. Even organic carbon contents of deeper layers showed no distinct difference between the upslope and mid-slope area colloids produced by the decomposition of litter, which was expected to contribute to the further migration of Cs into the deeper layer.
14.4 Conclusion
Cs preferred to adsorb to solid phases such as soil particles, and radioactive Cs emitted by the FDNPP were thought to have stayed near the surface soils. However, we observed a greater than 20 cm downward migration of radioactive Cs during the 2 years after the accident at our study site. The soil radioactive Cs content was highly correlated with the soil organic carbon content. Therefore, the dissolved and/or colloidal organic matter produced by the decomposition of the forest floor surface litter played a role in the enhanced migration of radioactive Cs. The mechanisms of the enhanced transport require further investigation.
References
Appelo CAJ, Postma D (2005) Chapter 6: Ion exchange. In: Geochemistry, groundwater and pollution, 2nd edn. Balkema, London
Comans RNJ, Haller M, de Preter P (1991) Sorption of cesium on Illite: non-equilibrium behavior and reversibility. Geochim Cosmochim Acta 55:433440
Contardi JS, Turner DR, Ahn TM (2001) Modeling colloid transport for performance assessment. J Contam Hydrol 47:323–333
Dalvaux B, Kruyts N, Cremer A (2000) Rhizospheric mobilization of radiocesium in soils. Environ Sci Technol 34:1489–1493
Ishikawa N, Uchida S, Tagami K (2007) Effects of clay minerals on radiocesium sorption behavior onto paddy field soil. Radioisotopes 56:519528
Kawahara T (1970) A study on the amount of mineral-nitrogen in forest soil (II) the relationships between mineralized nitrogen and total nitrogen, C/N and mineralized carbon. J Jpn For Soc 52(3):71–19
Kohatake S (1989) Estimation of the evapotranspiration rate by Thornthwaite or Harmon equation. Annu Disaster Prev Res Inst Kyoto Univ 32(2):309–317
Takahashi J, Onda Y, Hihara D, Tamura K (2018) Six-year monitoring of the vertical distribution of radiocesium in three forest soils after the Fukushima Dai-ichi nuclear power plant accident. J Environ Radioact 192:172–180
Tatsuno T, Hamamoto S, Nihei N, Nishimura T (2022) Vertical migration of cesium in weathered granite soil under flowing water condition depending on Cs concentration and states of dissolved organic matter. J Environ Manage 306:114409
Turner NB, Ryan JN, Saiers JE (2006) Effect of desorption on colloid-facilitated transport of contaminants: cesium, strontium, and illite colloids. Water Resour Res 42:W12S09. https://doi.org/10.1029/2006WR004972
Yamaguchi N, Takata Y, Hayashi K, Ishikawa S, Kuramata M, Eguchi S, Yoshikawa S, Sakaguchi A, Asada K, Wagai R, Makino T, Akahane I, Hiradat S (2012) Behavior of radiocaesium in soil-plant systems and its controlling factor: a review. Bull Natl Inst Agro Environ Sci 31:75–129
Acknowledgements
The authors would like to thank the Japan Society of Promotion Science for supporting this study (19H00958, 15H02467). To conduct field surveys for this study, we received help from the NPO Resurrection of Fukushima and former staff and students of the Laboratory of Soil Physics and Soil Hydrology, The University of Tokyo. Radioactivity measurements were conducted with support from the Isotope Facility for Agricultural Education and Research, The University of Tokyo.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Nishimura, T., Hamamoto, S., Yamasaki, T., Tatsuno, T. (2023). Decomposition of Organic Matters in a Forest Floor Enhanced Downward Migration of Radioactive Cs After the Accident of the FDNPP. In: Nakanishi, T.M., Tanoi, K. (eds) Agricultural Implications of Fukushima Nuclear Accident (IV). Springer, Singapore. https://doi.org/10.1007/978-981-19-9361-9_14
Download citation
DOI: https://doi.org/10.1007/978-981-19-9361-9_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9360-2
Online ISBN: 978-981-19-9361-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)