Abstract
Obesity is a rapidly rising problem worldwide in both developed and developing countries. It is not only reducing the quality of life but also shortens the duration of life with the comorbidities it brings [1]. Studies show that a two-point rise in the Body Mass Index (BMI) reduces one’s life expectancy by almost 10 years, and it also significantly affects the quality of life in morbidly obese patients [2]. Obesity is a serious medical problem as it links directly to many common comorbidities such as:
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Obesity is a rapidly rising problem worldwide in both developed and developing countries. It is not only reducing the quality of life but also shortens the duration of life with the comorbidities it brings [1]. Studies show that a two-point rise in the Body Mass Index (BMI) reduces one’s life expectancy by almost 10 years, and it also significantly affects the quality of life in morbidly obese patients [2]. Obesity is a serious medical problem as it links directly to many common comorbidities such as:
-
Type II diabetes mellitus.
-
Hypertension.
-
Coronary heart disease.
-
Hyperlipidemia.
-
Asthma.
-
Sleep apnea.
-
Reflux esophagitis.
-
Gallstones.
-
Osteoarthritis and spine problems.
-
Certain cancers, e.g., breast cancer.
There is no single effective treatment that fits all obese patients. Today, there are treatment options where behavioral therapies, medical treatments, endoscopic interventions, and surgical treatment options are applied alone or in combination. There is inconsistency in success rates and a high rate of regaining weight after treatments where nonsurgical weight-loss methods are applied alone or in combination [3]. Bariatric surgery (BS) often reduces premature mortality relative to morbidly obese individuals who have not undergone weight-loss intervention [4]. Therefore, surgical options are increasingly considered in the treatment of morbid obesity. Dietary modification, physiotherapy, drugs, and obesity surgery (if required) is the key approach.
Surgery for weight loss has been devised and practiced over the last 40 to 50 years. Bariatric surgical procedures cause weight loss by restricting the amount of food the stomach can hold, causing malabsorption of nutrients, or by a combination of both gastric restriction and malabsorption. Bariatric procedures also often cause hormonal changes. In this context the type of surgery falls into two broad categories:
-
Restrictive—reduce the size of the gastrointestinal tract, e.g., laparoscopic gastric banding, sleeve gastrectomy, vertical gastroplasty.
-
Malabsorptive—alter metabolism and reduce absorption, e.g., gastric bypass, biliopancreatic diversion, etc.
Bariatric or obesity surgery is recommended for the severely obese, in cases where weight reduction through medical therapy has been unsuccessful or where patients suffer from serious complications of obesity. According to the 1991 National Institutes of Health (NIH) consensus conference on gastrointestinal surgery for severe obesity [5], those who are suitable for obesity surgery are:
-
Patients with a Body Mass Index (BMI)* of more than 40 kg/m2.
-
Patients with a BMI* of more than 35 kg/m2 and obesity-related comorbidities.
While in the Asian population several studies have shown higher abdominal fat (5–10%) compared to others, so the Indication for Surgical treatment is 2.5–point BMI less:
-
Patients with a Body Mass Index (BMI)* of more than 37.5 kg/m2.
-
Patients with a BMI* of more than 32.5 kg/m2 with obesity-related comorbidities.
* BMI = weight (kg)/height (m) × height (m)
Surgery has become increasingly popular as they are usually performed via a laparoscopic approach. Several procedures like sleeve gastrectomy, gastric banding, and gastric bypass have been proven to be very effective not only in weight reduction but also in treating all comorbidities [6]. On average, patients can lose about 50–60% of their excess weight. More importantly, surgery can result in improvement or complete resolution of the various obesity complications like Diabetes mellitus type II, Hypertension, Obstructive Sleep Apnea, etc.
New guidelines on metabolic surgery in type II diabetes treatment algorithm have been published by international diabetes organizations due to the increasing data supporting the use of metabolic surgery for diabetes treatment [7]. Accordingly, they concluded that bariatric surgery should be recommended for patients with a BMI 40 kg/ m2 and those with inadequately controlled hyperglycemia and BMI 35 kg/m2 regardless of glycemic control. In addition, surgery should be considered for patients with a BMI of 30–34.9 kg/m2 and poorly controlled hyperglycemia, and for Asian patients with poorly controlled hyperglycemia with a BMI as low as 27.5 kg/m2 [7, 8].
Laparoscopic Gastric Banding (LAGB)
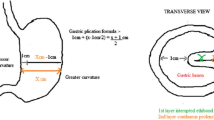
Gastric banding is a pure restrictive and reversible procedure and it is based on the principle of forming a small volume pouch near the stomach by wrapping the fundus with various synthetic grafts and limiting the passage to the distal part of the stomach. Food intake of patient is reduced by its restrictive and satiety effects. With LAGB, patients, experience early and prolonged satiety as well as reduced appetite. For this purpose, Wilkinson performed the first study on this subject in 1976 using the Marlex graft wrapped around the stomach [9]. Later, Hallberg and Forsell defined the device, which is now called the Swedish Adjustable Gastric Band (SAGB), in 1976 [10]. Also, during this period, an inflatable silicone-based gastric band, known today as the American Lap-Band, was defined by Kuzmak [11]. The first laparoscopic use of AGB was reported by Dr. Belachew in 1993 [12].
It is minimally invasive, and the diameter of the band is adjustable through an access port which is implanted under the skin. Adjustments of the band are usually carried out at an outpatient clinic during follow-up visits and are critical for successful outcomes.
On the technical point of view after the initial experience with Belachew’s original technique for band placement which is so-called perigastric technique, higher rate of complications like slippage and pouch dilatation were reported. Subsequently with the modified “pars flaccida technique” several studies and RCTS showed a significant reduction of these complications [13, 14].
Indications
The indications to undergo bariatric surgery are based on body mass index (BMI) as well as the presence of comorbidity.
-
BMI ≥ 40 kg/m2, or body weight ≥ 100lbs above ideal body weight.
-
BMI ≥35–<40 kg/m2 and ≥ 1 high-risk comorbid condition, or body weight ≥ 80lbs above ideal body weight + 1 comorbidity.
-
Failure to respond to, low likelihood of responding to, or refusal to undergo medically sound weight-loss program.
-
Well informed and motivated and accepts operative risk.
Contraindications
-
Absolute.
-
Mentally impaired, unable to weigh the risk and benefits of surgery.
-
Active neoplastic disease.
-
Cirrhosis with portal hypertension.
-
Unstable or incurable preexisting comorbidities (CAD, DM, asthma, AIDS, etc.), or uncontrolled psychiatric condition.
-
Pregnancy.
-
Immobility.
-
Inability or refusal to comply with postoperative regimens.
-
Active substance abuse.
-
Lack of social support.
-
Unable to tolerate general anesthesia.
-
-
Relative.
-
Age.
-
Coagulopathy.
-
Previous abdominal surgery.
-
Preoperative Preparations
Preoperative planning is very important in order to achieve a successful result in patients who have undergone bariatric surgery. A group of qualified medical professionals, such as psychiatrists/psychologists, nutritionists, cardiologists, pulmonologists, endocrinologists, surgeons, and social workers, are an integral part of patient optimization. Patients often attend group classes designed to educate them about lifestyle changes they should follow after surgery and what to expect during and after surgery. The technical details of the surgery and the physical changes that will occur, as well as the adaptation to the nutritional and psychological aspects of the surgery, are equally important. It is also important to examine patients before the operation in terms of excluding other diseases that may cause weight gain and to perform laboratory tests. Preoperative upper gastrointestinal system endoscopy is useful for the exclusion of gastric pathologies. At the same time, the presence of preoperative cholelithiasis should be evaluated with hepatobiliary ultrasound.
-
Patient education.
-
Psychological evaluation.
-
Thorough history and physical examination.
-
Referral to appropriate specialty.
-
Screening laboratory tests (FBC, liver function, HgbA1c, iron, total iron-binding capacity, vit B12, folate, vit D, calcium, thyroid function, serum lipid).
-
Gallbladder ultrasound.
-
Upper GI evaluation.
-
Dietary counseling.
-
Preoperative weight loss, esp. BMI >60 kg/m2.
OT Setup, Patient Position, and Operative Team Position (Fig.1)
-
Video monitor over at patient’s left and right shoulders.
-
Patient supine with arms out, preferably split leg, secured to operative table, reverse Trendelenburg (about 25°).
-
Surgeon between patient’s legs or on the patient’s right side if not split leg; assistant on either side of the patient or on the patient’s left side if not split leg.
Instrumentation
-
Optical Trocar or Veress needle for access.
-
5 mm ports (3 or 4)
-
15– 18 mm port (1)
-
30–45° scope, 5 or 10 mm (1)
-
Nathanson liver retractor or Snake Retractor (1).
-
Atraumatic graspers, 5 mm (2).
-
Maryland dissector, 5 mm (1).
-
Curved scissors, 5 mm (1).
-
Hook diathermy, 5 mm (1).
-
Energy-based scalpel (Thunderbeat, Olympus; Harmonic™ Ethicon, Ligasure™ Medtronic, etc.), 5 mm (1).
-
Goldfinger (Obtech, Ethicon) (1).
-
Band placer (1).
-
Needle holder (2).
-
Permanent sutures.
-
Gastric band with access port (1).
-
Suction/irrigation device (1).
Trocar/Port Placement (Fig. 2)
The places of incision and the number of trocars to be used are determined in a way that the surgeon feels comfortable. Generally, a total of four trocars are sufficient for this surgery. According to this;
-
Port 1
-
12–15 mm port
-
Midline 8–10 cm supra umbilical, placed optimally to view the operative field/working space (For initiation of working field, for the laparoscope/camera, for passage of gastric band).
-
-
Port 2
-
5 mm port
-
Just below the xiphoid for Nathanson liver retractor, or
-
Right Subcostal if a Snake Liver Retractor is utilized.
-
-
Port 3
-
5 mm or 10 mm port
-
Four finger breath below left costal margin, at the anterior axillary line (For left-hand assisting instruments).
-
-
Port 4
-
5 mm or 10 mm port
-
Below the right costal margin, at the anterior axillary line (For right-hand instruments).
-
Surgical Technique (Pars Flaccida Technique: (Figs. 3, 4, and 5)
-
Dissection at the Angle of His.
-
Retract the liver up and to the right with a Nathanson retractor, exposing the diaphragm at the esophageal hiatus.
-
Using graspers draw down the fundus.
-
Dissect the gastrophrenic peritoneal attachment to expose the left crus, using hook diathermy or energy-based scalpel (Harmonic™, Ligasure™).
-
-
Dissection at the Lesser Curve.
-
Draw the mid-lesser curve to the patient’s left, with graspers.
-
Divide the pars flaccida of the lesser omentum.
-
Retract the posterior wall of the lesser sac to expose the anterior margin of the right crus.
-
Make a small opening in the peritoneum about 5 mm in front of the anterior margin of the right crus.
-
Dissect the retroesophagogastric opening using a blunt instrument or articulating dissector “Goldfinger” (Obtech, Ethicon) until it exits at the left crus.
-
-
Band Placement and Calibration of Gastric Pouch (Figs. 6, 7, 8, and 9).
-
Band placer passed gently through the retroesophagogastric tunnel in a counterclockwise advancement until it exits at the left crus.
-
Band tubing is inserted into the slot of the placer.
-
Band placer withdrawn along its path to the lesser curve and retrieve the tubing.
-
Draw the tubing until the band is in place, and partially close the buckle.
-
Inflate the calibration balloon with 25 ml of air, withdraw the calibration tube until it touches the esophagogastric junction.
-
Position the band over the equator of the balloon.
-
Deflate the calibration balloon, and bring band to complete closure.
-
Anterior fixation of the fundus and anterior gastric wall over the band, with three to four ventro-ventral sutures.
-
Withdraw the calibration balloon.
-
-
Placement of Gastric Band Calibration Port.
-
Bring out band tubing through a port site, with a large loop remaining within to prevent the tube from ripping off the calibration port due to extensive movement of patient.
-
Connect band tubing to access port, and secure to anterior rectus sheath with permanent sutures.
-
Surgical Technique Descriptive
The patient is positioned in the anti-Trendelenburg position (20–30°) with a slight inclination to the right and legs apart. The endo-laparoscopic monitor is placed on the head of the patient. The operation is started with the surgeon between the legs of the patient and the assistant surgeon on the side of the patient. Pneumoperitoneum can be created either using a Veress needle Technique (at umbilicus or Palmer Point), open Hasson technique, or using an Optical Trocar for an easy access under vision. The 12–15 mm optical trocar is inserted on the midaxillary line four fingers below the left costal margin. Then, one 5 mm and two other 5–10 mm cannulas were inserted as in Fig. 2. Two are the working port and the right subcostal utilized for liver retraction if not a subxiphoid Nathanson Retractor is used. The left lobe of the liver is elevated to expose the cardia of the stomach and the diaphragmatic crus. The dissection starts from the greater curvature and continues towards the diaphragm, and, at this stage, the left paraesophageal ligament dissection is completed, and the left crus is exposed. Then, the pars flacida is opened and the peritoneal sheet close to the edge of the right crus is opened to enter the retrogastric area. A retrogastric tunnel is created using a “Goldfinger instrument” or an atraumatic grasper till reaching the left crus and the phrenogastric ligament. During this step, we avoid the use of calibrated tube or balloon to avoid injury of the posterior GE wall. The band is inserted and passed through the retrogastric tunnel and closed over the bucket, then secured by anterior gastro-gastric sutures using three or four nonabsorbable seromuscular stitches. This is to cover the anterior part of the band completely. If any injury or laceration of the posterior gastric wall is suspected, a methylene blue dye test is carried out. The connecting tube is passed through the subxiphoid port and connected to the port placed and anchored over the left rectus abdominis. The gastric band can be calibrated if needed after 3–4 weeks, with water/saline injection. Additional calibrations were later considered based on clinical evaluation of symptoms and weight loss during follow-up.
Postoperative Care
In order to keep the gastric band in the optimal position, it is very important to follow the patients with an appropriate diet program. The gastric passage may be narrowed due to postoperative edema of the gastric mucosa. Patients are started on the postoperative diet with liquids and continue with pureed, soft, and solid foods for a period of 3–4 weeks. These dietary guidelines should be given to patients in writing with the support of a dietician in the clinic. Patients may not be able to lose weight in this early period because the feeling of satiety caused by the band has not yet formed. When they start eating solids, they will often need reassurance that they will start losing weight [15].
-
Upper GI gastrografin study on the first postoperative day; if normal findings, patient allowed to take fluids then structured diet.
-
Adjustment of gastric band usually starts 4–6 weeks after operation and every 4–6 weeks thereafter based on the patient’s rate of weight loss and food-fluid tolerance.
-
Goal of gastric band adjustment.
-
Loss of excess weight within 18 months to 3 years.
-
Weight loss of 0.5–1.0 kg per week.
-
Sensation of prolonged satiety.
-
No negative symptoms.
-
-
Adjustment of Gastric Band (two different type).
-
SAGB (high volume, low pressure).
-
3–4 cc of fluid added at first adjustment
-
1–1.5 cc of fluid on subsequent adjustment
-
Final total volume of 6–8.5 cc.
-
-
LAP-BAND (low volume, high pressure).
-
0.5–1.0 cc of fluid added at first adjustment
-
0.3–0.5 cc of fluid on subsequent adjustment
-
Final total volume of 3–5 cc.
-
-
-
Adjustment Guidelines.
-
Adjustment not necessary.
-
Adequate rate of weight loss.
-
No negative symptoms.
-
Eating reasonable range of food.
-
-
Consider adding fluid.
-
Inadequate weight loss.
-
Rapid loss of satiety after meals.
-
Hunger between meals.
-
Increased volume of meals.
-
-
Consider removing fluid.
-
Vomiting, heartburn, reflux into the mouth.
-
Choking, coughing spells, wheezing; especially at night.
-
Difficulty with a broad range of food.
-
Maladaptive eating behavior.
-
-
Side-Effect and Complications after LAGB (Table 1)
Band patients require long-term follow-up and are likely to require adjustments to the band on a regular basis. Even in the experienced hands 10–20% of patients who have weight-loss operations require follow-up operations to correct complications [16]. The majority of revision surgeries are minor revisions due to minor complications such as port revision and repositioning. Abdominal hernias are the most common complications requiring follow-up surgery. More than one-third of obese patients who have gastric surgery develop gallstones [17]. During rapid or substantial weight loss a person’s risk of developing gallstones is increased. Gallstones can be prevented with supplemental bile salts taken for the first 6 months after surgery [18].
Nearly 30% of patients who have weight-loss surgery develop nutritional deficiencies such as anemia, osteoporosis, and metabolic bone disease. These deficiencies can be avoided if vitamin and mineral intake are maintained. Women of childbearing age should avoid pregnancy until their weight becomes stable because rapid weight loss and nutritional deficiencies can harm a developing fetus [19, 20].
Regarding the complications, LAGB is the obesity surgery with lowest rate of complications and mortality (0.2–0.4%) [21, 22]. The most common complications that require an intervention are band slippage, erosion and perforation, and port/tube dysfunction or infection.
Early complications are seen in the immediate postoperative period and include misplacement of the band, perforation, and early slippage with secondary acute pouch dilatation. Late complications include pouch dilatation, band herniation, spontaneous variation in volume, erosion of the gastric wall, and migration of the band.
Slippage
A gastric band can migrate distally along the stomach or the stomach proximally above the band. Most gastric band slippages are anterior and present chronically [23]. A posterior band slippage is rare but can occur if the gastric band has been placed within the lesser sac of the stomach. Misplacement of the band is usually caused by the surgeon’s lack of experience and rarely occurs when the surgeon is experienced. The band may be placed in the perigastric fat not a constant finding, and the diagnosis may be delayed for a few days. The use of barium has been controversial because it may cause inflammation and fibrosis in these critically ill patients or in the lower part of the stomach, the latter causing severe gastric outlet obstruction.
Perforation
As with any laparoscopic surgery, hollow organ perforations can be seen after LAGB, but specific to this procedure, perforations usually develop in the cardia of the stomach [24]. This early gastric perforation is usually due to surgical trauma to the stomach wall. The patient presents with fever, pain, and leukocytosis. Water-soluble contrast imaging may reveal the leakage from the stomach. However, leakage is not a constant finding, and the diagnosis may be delayed for a few days. The use of barium has been controversial because it may cause inflammation and fibrosis in these critically ill patients and is probably better avoided if there is definite evidence of leakage. Gastrografin is an alternative option. CT is also diagnostic, showing the leakage and the possible associated subphrenic abscess.
Pouch Dilation
Early pouch dilatation has been described in low-positioned bands. Pouch dilatation is also a common late complication. After surgery, the pouch gradually increases in volume but retains a grossly concentric shape. It may also be secondary to overinflation of the band or to eccentric band herniation that results from focal band weakness. A contrast X-ray swallow test will identify the gastric pouch enlargement, diagnostic of a pouch dilatation. Management of a pouch dilatation should consist of initial band deflation with the pars flaccida approach, where with minimal dissection and higher position of the band, there is less risk for dilation. Slippage of the band can cause eccentric pouch dilation [25].
Erosion
The clinical presentation of chronic gastric erosion varies between asymptomatic conditions and acute abdominal emergency. Mechanical damage to the wall may be secondary to intraoperative trauma to the muscular layers, inflammatory reaction to foreign bodies, infection, and use of nonsteroidal anti-inflammatory medication. it is eventually a consequence of local gastric ischemia secondary to a tight band and the incidence of erosion following gastric band surgery remains currently at around 1% [26]. The passage of the contrast out of the lumen around the band is a certain indication of band erosion. Gastric erosion is highly likely if an open band is seen. Findings may be associated with a change in band position.
Leakage of the Banding System
Leakage is typically a late complication. It may occur at the level of the band or the connector tube or at the access port. It is first suspected when filling and insufficient deflating volume of the banding system combined with loss of eating restriction are observed Leakage of contrast material is usually detected while adjusting the band diameter. The incidence of port-related revisions is around 6% and the majority of these are for the management of leaks [27].
Infection
As around any foreign body, soft-tissue infection around the access port is possible. In addition, even the sterile puncture and adjustment of the stoma size may introduce infection, which then extends along the connector tube and along the band, with possible abscess formation. Infection increases the risk of perforation and fistulization and may necessitate surgical debridement and removal of the band.
Since its introduction in 1993, laparoscopic adjustable gastric banding has been the subject of many studies and evaluations. The continuous progress in surgical technique and increasing experience of surgeons have decreased the rate of many complications. LAGB procedure has been a very popular procedure for a while due to the relatively low learning curve, being technically easy, the duration of hospitalization is short, it can be applied as outpatient operations in some places, the early complication rates are low, and the desired level of weight loss can be achieved due to the adjustment of the band [28, 29]. The popularity of Gastric Banding was at the peak around 2008–2010 (about 40% of bariatric procedures worldwide) and then due to the high number of long-term and serious complications such as weight gain, obstructive symptoms, dysphagia, band slippage, esophageal dilatation, esophagitis, and gastric erosion and also the advent of other restrictive procedures like sleeve gastrectomy the frequency of LAGB procedures went down dramatically worldwide [30, 31]. Many patients and surgeons today prefer procedures like Laparoscopic Sleeve Gastrectomy or Roux-en-Y gastric bypass as an alternative to gastric banding. However, even though results have shown the efficacy of the banding in weight loss, controlling comorbidities such as diabetes mellitus II, hypertension, and OSA when the long-term results showed failure in weight loss, weight regain, long-term complications, banding becomes less and less utilized today [25, 28, 32, 33].
References
Gundogdu E, Moran M. Adjustable gastric banding. Ann Laparosc Endosc Surg. 2020. https://doi.org/10.21037/ales-2019-bms-06.
Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, Buchwald H, Scopinaro N. IFSO worldwide survey 2016: primary, Endoluminal, and Revisional procedures. Obes Surg. 2018;28(12):3783–94. https://doi.org/10.1007/s11695-018-3450-2.
Bond DS, Phelan S, Leahey TM, Hill JO, Wing RR. Weight-loss maintenance in successful weight losers: surgical vs non-surgical methods. Int J Obes. 2009;33(1):173–80. https://doi.org/10.1038/ijo.2008.256.
Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg. 2004;199(4):543–51. https://doi.org/10.1016/j.jamcollsurg.2004.06.014.
NIH conference. Gastrointestinal surgery for severe obesity. Consensus development conference panel. Ann Intern Med. 1991;115(12):956–61.
Golzarand M, Toolabi K, Farid R. The bariatric surgery and weight losing: a meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surg Endosc. 2017;31(11):4331–45. https://doi.org/10.1007/s00464-017-5505-1. Epub 2017 Apr 4.
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE, Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77. https://doi.org/10.2337/dc16-0236.
American Diabetes Association. 7. Obesity management for the treatment of type 2 diabetes. Diabetes Care. 2017;40(Suppl 1):S57–63. https://doi.org/10.2337/dc17-S010.
Wilkinson LH, Peloso OA. Gastric (reservoir) reduction for morbid obesity. Arch Surg. 1981;116(5):602–5. https://doi.org/10.1001/archsurg.1981.01380170082014.
Hallberg D, Forsell I. Ballongband vid behandling av massiv overvikt (balloon band for the treatment of massive obesity). Svensk Kirwgi. 1985:43–106.
Kuzmak LI. Silicone gastric banding: a simple and effective operation for morbid obesity. Contemp Surg. 1986;28:13–8.
Belachew M, Legrand M, Vincenti VV, Deffechereux T, Jourdan JL, Monami B, Jacquet N. Laparoscopic placement of adjustable silicone gastric band in the treatment of morbid obesity: how to do it. Obes Surg. 1995;5(1):66–70. https://doi.org/10.1381/096089295765558196.
O'Brien PE, Dixon JB, Laurie C, Anderson M. A prospective randomized trial of placement of the laparoscopic adjustable gastric band: comparison of the perigastric and pars flaccida pathways. Obes Surg. 2005;15(6):820–6. https://doi.org/10.1381/0960892054222858.
Di Lorenzo N, Furbetta F, Favretti F, et al. Laparoscopic adjustable gastric banding via pars flaccida versus perigastric positioning: technique, complications, and results in 2,549 patients. Surg Endosc. 2010;24(7):1519–23. https://doi.org/10.1007/s00464-009-0669-y.
Cobourn Chris S, Dixon JB. LAGB: the technique. In: Obesity, bariatric and metabolic surgery. Cham: Springer; 2016. p. 299–306.
Carelli AM, Youn HA, Kurian MS, Ren CJ, Fielding GA. Safety of the laparoscopic adjustable gastric band: 7-year data from a U.S. center of excellence. Surg Endosc. 2010;24(8):1819–23. https://doi.org/10.1007/s00464-009-0858-8.
Gustafsson U, Benthin L, Granström L, Groen AK, Sahlin S, Einarsson C. Changes in gallbladder bile composition and crystal detection time in morbidly obese subjects after bariatric surgery. Hepatology. 2005 Jun;41(6):1322–8. https://doi.org/10.1002/hep.20686.
Uy MC, Talingdan-Te MC, Espinosa WZ, Daez ML, Ong JP. Ursodeoxycholic acid in the prevention of gallstone formation after bariatric surgery: a meta-analysis. Obes Surg. 2008;18(12):1532–8. https://doi.org/10.1007/s11695-008-9587-7.
Dixon JB, Dixon ME, O'Brien PE. Pregnancy after lap-band surgery: management of the band to achieve healthy weight outcomes. Obes Surg. 2001;11(1):59–65. https://doi.org/10.1381/096089201321454123.
Jefferys AE, Siassakos D, Draycott T, Akande VA, Fox R. Deflation of gastric band balloon in pregnancy for improving outcomes. Cochrane Database Syst Rev. 2013;4:CD010048. https://doi.org/10.1002/14651858.CD010048.pub2.
Chakravarty PD, McLaughlin E, Whittaker D, Byrne E, Cowan E, Xu K, Bruce DM, Ford JA. Comparison of laparoscopic adjustable gastric banding (LAGB) with other bariatric procedures; a systematic review of the randomised controlled trials. Surgeon. 2012;10(3):172–82. https://doi.org/10.1016/j.surge.2012.02.001. Epub 2012 Mar 8.
O'Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257(1):87–94. https://doi.org/10.1097/SLA.0b013e31827b6c02.
Boschi S, Fogli L, Berta RD, Patrizi P, Di Domenico M, Vetere F, Capizzi D, Capizzi FD. Avoiding complications after laparoscopic esophago-gastric banding: experience with 400 consecutive patients. Obes Surg. 2006;16(9):1166–70. https://doi.org/10.1381/096089206778392329.
Soto FC, Szomstein S, Higa-Sansone G, Mehran A, Blandon RJ, Zundel N, Rosenthal RJ. Esophageal perforation during laparoscopic gastric band placement. Obes Surg. 2004;14(3):422–5. https://doi.org/10.1381/096089204322917981.
O'Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, Crosthwaite G, Brown W. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-Centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3–14. https://doi.org/10.1007/s11695-018-3525-0.
Brown WA, Egberts KJ, Franke-Richard D, Thodiyil P, Anderson ML, OʼBrien PE. Erosions after laparoscopic adjustable gastric banding: diagnosis and management. Ann Surg. 2013;257(6):1047–52. https://doi.org/10.1097/SLA.0b013e31826bc21b.
Tog CH, Halliday J, Khor Y, Yong T, Wilkinson S. Evolving pattern of laparoscopic gastric band access port complications. Obes Surg. 2012;22(6):863–5. https://doi.org/10.1007/s11695-011-0567-y.
Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–87. https://doi.org/10.1001/jamasurg.2013.3654.
Chapman AE, Kiroff G, Game P, Foster B, O'Brien P, Ham J, Maddern GJ. Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic literature review. Surgery. 2004;135(3):326–51. https://doi.org/10.1016/S0039-6060(03)00392-1.
Gündoğdu E, Bilgiç Cİ, Moran M, Güldoğan CE, Dilektaşli E, Özmen MM. Evaluation of the effects of laparoscopic adjustable gastric banding versus laparoscopic sleeve gastrectomy on weight loss. Eur Res J. 2019;6(1):36–42.
English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259–63. https://doi.org/10.1016/j.soard.2017.12.013. Epub 2017 Dec 16.
Himpens J, Cadière GB, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146(7):802–7. https://doi.org/10.1001/archsurg.2011.45.
Chevallier JM, Zinzindohoué F, Douard R, Blanche JP, Berta JL, Altman JJ, Cugnenc PH. Complications after laparoscopic adjustable gastric banding for morbid obesity: experience with 1,000 patients over 7 years. Obes Surg. 2004;14(3):407–14. https://doi.org/10.1381/096089204322917954.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Lomanto, D., Gundogdu, E., Ozmen, M.M. (2023). Laparoscopic Gastric Banding for Morbid Obesity. In: Lomanto, D., Chen, W.TL., Fuentes, M.B. (eds) Mastering Endo-Laparoscopic and Thoracoscopic Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-19-3755-2_40
Download citation
DOI: https://doi.org/10.1007/978-981-19-3755-2_40
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3754-5
Online ISBN: 978-981-19-3755-2
eBook Packages: MedicineMedicine (R0)