Abstract
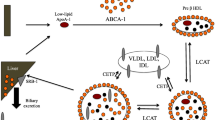
Sepsis has been recognized as a global health burden in the year 2017 for its high morbidity and mortality. HDL, a cholesterol transporter, also plays vital role in inflammation besides its typical role in reverse cholesterol transportation. In septic patients, HDL levels dropped significantly. HDL exerts a variety of protective effects in the pathophysiology of sepsis including acting on pathogens, inhibiting macrophage inflammatory reaction, and modulating endothelial function in sepsis. Studies have shown that rHDL or apoA-I mimetic peptides could be therapeutic potentials in sepsis. HDL has caused increasing concern as a potential therapeutic agent.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Reinhart K et al (2017) Recognizing sepsis as a Global Health priority - a WHO resolution. N Engl J Med 377(5):414–417
Angus DC et al (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310
Mayr FB, Yende S, Angus DC (2014) Epidemiology of severe sepsis. Virulence 5(1):4–11
Fleischmann C et al (2016) Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 193(3):259–272
Singer M et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810
Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369(9):840–851
Vincent JL et al (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34(2):344–353
Vincent JL et al (2014) Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2(5):380–386
Vincent JL et al (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302(21):2323–2329
Karlsson S et al (2007) Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med 33(3):435–443
Boyd JH, Russell JA, Fjell CD (2014) The meta-genome of sepsis: host genetics, pathogens and the acute immune response. J Innate Immun 6(3):272–283
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348(2):138–150
Cecconi M et al (2018) Sepsis and septic shock. Lancet 392(10141):75–87
Ferrer R et al (2014) Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 42(8):1749–1755
Garnacho-Montero J et al (2008) Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study. J Antimicrob Chemother 61(2):436–441
Ibrahim EH et al (2000) The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118(1):146–155
van Leeuwen HJ et al (2003) Lipoprotein metabolism in patients with severe sepsis. Crit Care Med 31(5):1359–1366
Tanaka S et al (2017) Low HDL levels in sepsis versus trauma patients in intensive care unit. Ann Intensive Care 7(1):60
Chien JY et al (2005) Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med 33(8):1688–1693
Barlage S et al (2009) Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med 35(11):1877–1885
Genga KR et al (2018) CETP genetic variant rs1800777 (allele A) is associated with abnormally low HDL-C levels and increased risk of AKI during sepsis. Sci Rep 8(1):16764
Cirstea M et al (2017) Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care 38:289–294
Pirillo A, Catapano AL, Norata GD (2019) Biological consequences of dysfunctional HDL. Curr Med Chem 26(9):1644–1664
Guirgis FW et al (2018) HDL cholesterol efflux is impaired in older patients with early sepsis: a subanalysis of a prospective pilot study. Shock 50(1):66–70
Guirgis FW et al (2017) Exploring the predictive ability of dysfunctional high-density lipoprotein for adverse outcomes in emergency department patients with sepsis: a preliminary investigation. Shock 48(5):539–544
Guirgis FW et al (2016) Cholesterol levels and long-term rates of community-acquired sepsis. Crit Care 20(1):408
Rall DP, Gaskins JR, Kelly MG (1957) Reduction of febrile response to bacterial polysaccharide following incubation with serum. Am J Phys 188(3):559–562
Ulevitch RJ, Johnston AR, Weinstein DB (1979) New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest 64(5):1516–1524
Ulevitch RJ, Johnston AR, Weinstein DB (1981) New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest 67(3):827–837
Flegel WA et al (1989) Inhibition of endotoxin-induced activation of human monocytes by human lipoproteins. Infect Immun 57(7):2237–2245
Dai L et al (2010) The apolipoprotein A-I mimetic peptide 4F prevents defects in vascular function in endotoxemic rats. J Lipid Res 51(9):2695–2705
Lamping N et al (1998) LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J Clin Invest 101(10):2065–2071
Vesy CJ et al (2000) Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from gram-negative bacterial membranes. Infect Immun 68(5):2410–2417
Levels JH et al (2005) Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect Immun 73(4):2321–2326
Cohen J (1999) Adjunctive therapy in sepsis: a critical analysis of the clinical trial programme. Br Med Bull 55(1):212–225
Hoekstra M, Sorci-Thomas M (2017) Rediscovering scavenger receptor type BI: surprising new roles for the HDL receptor. Curr Opin Lipidol 28(3):255–260
Cai L et al (2008) SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest 118(1):364–375
Vishnyakova TG et al (2003) Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J Biol Chem 278(25):22771–22780
De Kimpe SJ et al (1995) The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci U S A 92(22):10359–10363
Levels JH et al (2003) Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect Immun 71(6):3280–3284
Grunfeld C et al (1999) Lipoproteins inhibit macrophage activation by lipoteichoic acid. J Lipid Res 40(2):245–252
Jiao YL, Wu MP (2008) Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine 43(1):83–87
Kumar V (2018) Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int Immunopharmacol 58:173–185
De Nardo D et al (2014) High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 15(2):152–160
Huang J et al (2018) AMPK regulates immunometabolism in sepsis. Brain Behav Immun 72:89–100
Vachharajani VT et al (2014) SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol 96(5):785–796
Feig JE et al (2011) HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A 108(17):7166–7171
Cockerill GW et al (1995) High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol 15(11):1987–1994
McDonald MC et al (2003) Reconstituted high-density lipoprotein attenuates organ injury and adhesion molecule expression in a rodent model of endotoxic shock. Shock 20(6):551–557
Park SH et al (2003) Involvement of transcription factors in plasma HDL protection against TNF-alpha-induced vascular cell adhesion molecule-1 expression. Int J Biochem Cell Biol 35(2):168–182
Yuhanna IS et al (2001) High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 7(7):853–857
Mineo C et al (2003) High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem 278(11):9142–9149
Martin G et al (2014) Endothelial (NOS3 E298D) and inducible (NOS2 exon 22) nitric oxide synthase polymorphisms, as well as plasma NOx, influence sepsis development. Nitric Oxide 42:79–86
Spiller F et al (2019) Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide 89:32–40
Norata GD et al (2004) HDL3 induces cyclooxygenase-2 expression and prostacyclin release in human endothelial cells via a p38 MAPK/CRE-dependent pathway: effects on COX-2/PGI-synthase coupling. Arterioscler Thromb Vasc Biol 24(5):871–877
Liu D et al (2011) Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1. Am J Physiol Cell Physiol 301(3):C739–C748
Zhang QH et al (2012) An involvement of SR-B1 mediated PI3K-Akt-eNOS signaling in HDL-induced cyclooxygenase 2 expression and prostacyclin production in endothelial cells. Biochem Biophys Res Commun 420(1):17–23
Viswambharan H et al (2004) Reconstituted high-density lipoprotein inhibits thrombin-induced endothelial tissue factor expression through inhibition of RhoA and stimulation of phosphatidylinositol 3-kinase but not Akt/endothelial nitric oxide synthase. Circ Res 94(7):918–925
Guo L et al (2013) High density lipoprotein protects against polymicrobe-induced sepsis in mice. J Biol Chem 288(25):17947–17953
Yan YJ et al (2006) Beneficial effects of ApoA-I on LPS-induced acute lung injury and endotoxemia in mice. Life Sci 79(2):210–215
Parolini C (2020) A compendium of the biological effects of apolipoprotein A-IMilano. J Pharmacol Exp Ther 372(1):54–62
Zhang X, Wang L, Chen B (2015) Recombinant HDL (Milano) protects endotoxin-challenged rats from multiple organ injury and dysfunction. Biol Chem 396(1):53–60
Levine DM et al (1993) In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci U S A 90(24):12040–12044
Zhang Z et al (2009) Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol 297(2):H866–H873
Moreira RS et al (2014) Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am J Physiol Regul Integr Comp Physiol 307(5):R514–R524
Kwon WY et al (2012) 4F, apolipoprotein AI mimetic peptide, attenuates acute lung injury and improves survival in endotoxemic rats. J Trauma Acute Care Surg 72(6):1576–1583
Tanaka S et al (2020) Reconstituted high-density lipoprotein therapy improves survival in mouse models of sepsis. Anesthesiology 132(4):825–838
Pajkrt D et al (1996) Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med 184(5):1601–1608
Pajkrt D et al (1997) Differential effects of reconstituted high-density lipoprotein on coagulation, fibrinolysis and platelet activation during human endotoxemia. Thromb Haemost 77(2):303–307
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Cao, H., Huang, W. (2022). HDL and Sepsis. In: Zheng, L. (eds) HDL Metabolism and Diseases. Advances in Experimental Medicine and Biology, vol 1377. Springer, Singapore. https://doi.org/10.1007/978-981-19-1592-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-1592-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1591-8
Online ISBN: 978-981-19-1592-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)