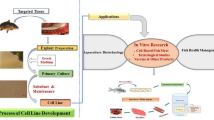
Abstract
Cell culture serves as a reliable and proficient tool in diverse research fields such as virology, physiology, toxicology, immunology, oncology, genetics, and pharmacology. These systems can be employed for pathogen detection, confirmation, and characterization especially of viruses. It is also applicable in the case of intracellular bacteria, myxosporean or microsporean parasites as well. Fish cell cultures have gained more popularity in recent years and have prominent roles in viral disease diagnosis. Since treatment options are limited for many viral diseases, early disease diagnosis and proactive management measures are key for successful fish health management. The ability to propagate fish viruses in vitro using cell cultures is imperative in advancing research on viruses and to facilitate disease management strategies such as vaccines and antiviral agents. Moreover, potential host range of pathogens via susceptibility to cell cultures, virus-host cell interactions, and virus localization studies using cell cultures provide a better understanding of the viral pathogenesis. Availability of suitable fish cell cultures for propagation of viruses and disease diagnosis is very limited, which is a major concern in this area. The wide array of applications exemplifies the versatility, cost-effectiveness, and high potential of fish cell cultures in various research fields. The recent swift growth observed in research employing cell cultures is definitely an outcome of the progress in this sector and also due to increasing ethical demands for reduction and replacement of animal use in research. In the near future, innovations in 3D cell culture and CRISPER-Cas9 genome editing will further enhance the research prospects of fish cell culture systems.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Aarattuthodiyil S, Dharan V (2019) Viruses impacting the catfish industry. NWAC News 16(1):10–11
Aarattuthodiyil S, Griffin MJ, Greenway T, Khoo L, Byars T, Wise DJ (2020) An orally delivered, live-attenuated Edwardsiella ictaluri vaccine efficiently protects channel catfish fingerlings against multiple Edwardsiella ictaluri field isolates. J World Aquac Soc 51(6):1354–1372
Ahmed IVP, Chandra V, Parameswaran V, Venkatesan C, Shukla R, Bhonde RR, Hameed AS (2008) A new epithelial-like cell line from eye muscle of catla (Catla catla): development and characterization. J Fish Biol 72:2026–2038
Ahmed VPI, Babu VS, Chandra V, Nambi KSN, Thomas J, Ramesh B, Sahul Hameed AS (2009) A new fibroblastic-like cell line from heart muscle of the Indian major carp (Catla catla): development and characterization. Aquaculture 293:180–186
Alge C, Hauck S, Priglinger S, Kampik A, Ueffing M (2006) Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival. J Proteome Res 5(4):862–878
Allen DD, Caviedes R, Cárdenas A, Shimahara T, Segura-Aguilar J, Caviedes P (2005) Cell lines as in vitro models for drug screening and toxicity studies. Drug Dev Ind Pharm 31:757–768
Altman PL, Dittmer DS (1972) Biology data book, vol 1, 2nd edn. Federation of American Societies for Experimental Biology, Bethesda, MD, p 519
Arango MT, Quintero-Ronderos P, Castiblanco J, Montoya-Ortíz G (2013) Cell culture and cell analysis (Chapter 45). In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A et al (eds) Autoimmunity: from bench to bedside. El Rosario University Press, Bogota, Colombia
Arnizaut AB, Hanson LA (2011) Antibody response of channel catfish after channel catfish virus infection and following dexamethasone treatment. Dis Aquat Org 95:189–201
Ashton I, Clements K, Barrow SE, Secombes CJ, Rowley AF (1994) Effects of dietary fatty acids on eicosanoid generating capacity, fatty acid composition and chemotactic activity of rainbow trout (Oncorhynchus mykiss) leucocytes. Biochim Biophys Acta 1214:253–262
Babich H, Borenfreund E (1991) Cytotoxicity and genotoxicity assays with cultured fish cells: a review. Toxicol In Vitro 5(1):91–100
Babich H, Rosenberg DW, Borenfreund E (1991) In vitro cytotoxicity studies with the fish hepatoma cell line, plhc-1. Ecotoxicol Environ Saf 21(3):327–336
Bahia H, Ashman JNE, Cawkwell L, Lind M, Monson JRT, Drew PJ, Greenman J (2002) Karyotypic variation between independently cultured strains of the cell line MCF-7 identified by multicolour fluorescence in situ hybridization. Int J Oncol 20:489–494
Bailey G, Taylor M, Selivonchick D, Eisele T, Hendricks J, Nixon J, Pawlowski N, Sinnhuber R (1982) Mechanisms of dietary modification of aflatoxin B1 carcinogenesis. Basic Life Sci 21:149–165
Ballester M, Bolonio M, Santamaria R (2019) Direct conversion of human fibroblast to hepatocytes using a single inducible polycistronic vector. Stem Cell Res Ther 10:317
Balls M, Riddell RJ, Worden AN (1983) Animals and alternatives in toxicity testing. Academic Press, London, pp 175–184
Balmer BF, Powers RL, Zhang TH, Lee J, Vigant F, Lee B, Jung ME, Purcell MK, Snekvik K, Aguilar HC (2017) Inhibition of an aquatic rhabdovirus demonstrates promise of a broad-spectrum antiviral for use in aquaculture. J Virol 91
Balmer BF, Getchell RG, Powers RL, Lee J, Zhang T, Jung ME, Purcell MK, Snekvik K, Aguilar H (2018) Broad-spectrum antiviral JL122 blocks infection and inhibits transmission of aquatic rhabdoviruses. Virology 525:143–149
Bang FB (1960) Virus disease: some aspects of host and tissue specificity. Annu Rev Med 11:1–18
Baron S, Fons M, Albrecht T (1996) Viral pathogenesis. Medical microbiology, 4th edn. University of Texas Medical Branch, Galveston
Beale AJ (1981) Cell substrate for killed poliovaccine production. Dev Biol Standard 47:19–23
Bedrnik P, Vavra J (1972) Further observations on the maintenance of Encephalitozoon cuniculi in tissue culture. J Protozool 19:75S
Behrens A, Schirmer K, Bols NC, Segner H (2001) Polycyclic aromatic hydrocarbons as inducers of cytochrome P4501A enzyme activity in the rainbow trout liver cell line, RTL-W1 and in primary cultures of rainbow trout hepatocytes. Environ Toxicol Chem 20:632–643
Benjaminson MA, Gilchriest JA, Lorenz M (2002) In vitro edible muscle protein production system (MPPS): stage 1, fish. Acta Astronaut 51(12):879–889
Bermejo-Nogales A, Fernández-Cruz ML, Navas JM (2017) Fish cell lines as a tool for the ecotoxicity assessment and ranking of engineered nanomaterials. Regul Toxicol Pharmacol 90:297–307
Bibila TA, Ranucci CS, Glazomitsky K, Buckland BC, Aunins JG (1994) Monoclonal antibody process development using medium concentrates. Biotechnol Prog 10:87–96
Bols NC, Lee LEJ (1991) Technology, and uses of cell culture from tissues and organs of bony fish. Cytotechnology 6:163–187
Bols NC, Yip JHK, Wolf BR (1984) Trout red blood cells treated with proteases fuse when placed on glass slides. Biosci Rep 4:65–70
Borenfreund E, Puerner JA (1985) A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J Tissue Cult Methods 9:7–9
Bouchard B, Fuller BB, Vijayasaradhi S, Houghton AN (1989) Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J Exp Med 169:2029–2042
Bowser PR, Munson AD (1986) Seasonal variation in channel catfish virus antibody titers in adult channel catfish. Prog Fish Cult 48:198–199
Bowser PR, Plumb JA (1980) Channel catfish virus: comparative replication and sensitivity of cell lines from channel catfish ovary and the brown bullhead. J Wildl Dis 16(3):451–454
Buchmann K, Nielsen CV, Bresciani J (2000) In vitro interactions between epithelial cells and Gyrodactylus derjavini. J Helminthol 74:203–208
Burdall SE, Hanby AM, Lansdown MR et al (2003) Breast cancer cell lines: friend or foe? Breast Cancer Res 5:89. https://doi.org/10.1186/bcr577
Burki R, Vermeirssen E, Körner O, Joris C, Burkhardt-Holm P, Segner H (2006) Assessment of estrogenic exposure in Brown Trout (Salmo trutta) in a Swiss Midland River: integrated analysis of Passive Samplers, Wild and Caged Fish, and Vitellogenin MRNA and Protein. Environ Toxicol Chem 25:2077–2086
Caminada D, Zaja R, Smital T, Fent K (2008) Human pharmaceuticals modulate P-gp1 (ABCB1) transport activity in the fish cell line PLHC-1. Aquat Toxicol 90(3):214–222
Capstick PB, Telling RC, Chapman WG, Stewart DL (1962) Growth of a cloned strain of hamster kidney cells in suspended cultures and their susceptibility to the virus of foot and mouth disease. Nature 195:1163–1164
Carrel A, Burrows MT (1911) Cultivation of tissues in vitro and its technique. J Exp Med 13(3):387–396
Castaño A, Cantarino MJ, Castillo P, Tarazona JV (1996) Correlations between the RTG-2 cytotoxicity test EC50 and in vivo LC50 rainbow trout bioassay. Chemosphere 32:2141–2157
Castaño A, Bols N, Braunbeck T, Dierickx P, Halder M, Isomaa B, Kawahara K, Lee L, Mothersill C, Pärt P, Repetto G, Riego Sintes J, Rufli H, Smith R, Wood C, Segner H (2003) The use of fish cells in ecotoxicology: the report and recommendations of ECVAM Workshop 47. Altern Lab Anim: ATLA 31:317–351. https://doi.org/10.1177/026119290303100314
Chacon E, Acosta D, Lemasters JJ (1997) Primary cultures of cardiac myocytes as in vitro models for pharmacological and toxicological assessments. In: In vitro methods in pharmaceutical research, vol 9. The University Press, Cambridge, pp 209–223
Chang N, Sun C, Gao L, Zhu D, Xu X, Zhu X, Xiong JW, Xi JJ (2013) Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res 23:465–472
Chen SN, Kou GH (1987) Establishment, characterization and application of 14 cell lines from warm-water fish. In: Kuroda Y, Kurstak E, Maramorosch K (eds) Invertebrate and fish tissue culture. Japan Scientific Societies Press, Tokyo, pp 218–227
Chen MJ, Chiou PP, Liao YH, Lin CM, Chen TT (2010) Development and characterization of five rainbow trout pituitary single-cell clone lines capable of producing pituitary hormones. J Endocrinol 205(1):69–78
Chen B, Zheng Z, Yang J, Chi H, Huang H, Gong H (2019) Development and characterization of a new cell line derived from European eel Anguilla anguilla kidney. Biol Open 8(1):bio037507. https://doi.org/10.1242/bio.037507
Chiou P, Bols N, Douglas S, Chen TT (2006) Regulation of immune-relevant genes in the trout macrophage cell line RTS11 by antimicrobial peptides. Dev Comp Immunol 30:797–806
Church JE, Hodgson WC (2002) The pharmacological activity of fish venoms. Toxinology 40(8):1083–1093
Ciarlo CA, Zon LI (2016) Embryonic cell culture in zebrafish. Methods Cell Biol 133:1–10
Clem LW, Moewus L, Sigel M (1961) Studies with cells from marine fish in tissue culture. Proc Soc Exp Biol Med 108:762–766
Collet B, Collins C, Lester K (2018) Engineered cell lines for fish health research. Dev Comp Immunol 80:34–40
Collodi P, Kamei Y, Ernst T, Miranda C, Buhler DR, Barnes DW (1992) Culture of cells from zebrafish (Brachydanio rerio) embryo and adult tissues. Cell Biol Toxicol 8(1):43–61
Cossarini-Dunier M (1987) Effects of the pesticide atrazine, lindane, and of manganese ions on cellular immunity of carp, Cyprinus carpio. J Fish Biol 31:67–73
Cossarini-Dunier M, Hattenberger AM (1988) Effects of pesticides (atrazine and lindane) on the replication of spring viremia of carp virus. Ann Rech Vet 19:209–211
Davison AJ, Eberle R, Ehlers B, Hayward G, McGeoch D, Minson A, Pellett P, Roizman B, Studdert M, Thiry E (2009) The order herpesvirales. Arch Virol 154(1):171–177
de Sena J, Rio GJ (1975) Partial purification and characterization of RTG-2 fish cell interferon. Infect Immun 11(4):815–822
Dehler CE, Boudinot P, Martin SA, Collet B (2016) Development of an efficient genome editing method by CRISPR/Cas9 in a fish cell line. Mar Biotechnol 18(4):449–452
Desoize B, Gimonet D, Jardiller JC (1998) Cell culture as spheroids: an approach to multicellular resistance. Anticancer Res 18(6a):4147–4158
Desportes-Livage I, Chilmonczyk S, Hedrick R, Ombrouck C, Monge D, Maiga I, Gentilini M (1996) Comparative development of two microsporidian species: Enterocytozoon bieneusi and Enterocytozoon salmonis, reported in AIDS patients and salmonid fish, respectively. J Eukaryot Microbiol 43(1):49–60
Dharan V, Khoo L, Phelps NB, Kumar G, Steadman J, Bosworth B, Aarattuthodi S (2021) An investigation into the pathogenesis of blue catfish alloherpesvirus in ictalurid catfish. J World Aquacult Soc. https://doi.org/10.1111/jwas.12850
Dishon A (2009) Back passage/shed assay for cyprinid herpes virus type 3 modified live virus according to VS Memorandum No. 800.201. Kovax Ltd
Dixon P (1988) Immunization with viral antigens: viral diseases of carp and catfish. Dev Biol Stand 90:221–232
Driever W, Rangini Z (1993) Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell Dev Biol 29A(9):749–754
Dumont J, Euwart D, Mei B, Estes S, Kshirsagar R (2016) Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit Rev Biotechnol 36(6):1110–1122
Etoh H, Suyama I, Hyodo-Taguchi Y, Matsudaira H (1987) Establishment and characteristics of various cell lines from medaka (Teleostei). In: Kuroda Y, Kurstak E, Maramorosch K (eds) Invertebrate and fish tissue culture. Japan Scientific Societies Press, Tokyo, pp 266–269
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Faber MN, Sojan JM, Saraiva M, van West P, Secombes CJ (2021) Development of a 3D spheroid cell culture system from fish cell lines for in vitro infection studies: evaluation with Saprolegnia parasitica. J Fish Dis 44(6):701–710. https://doi.org/10.1111/jfd.13331
Fent K (2001) Fish cell lines as versatile tools in ecotoxicology: assessment of cytotoxicity, cytochrome P4501A induction potential and estrogenic activity of chemicals and environmental samples. Toxicol In Vitro 15(4-5):477–488
Fierro-Castro C, Barrioluengo L, López-Fierro P, Razquin BE, Villena AJ (2013) Fish cell cultures as in vitro models of inflammatory responses elicited by immunostimulants. Expression of regulatory genes of the innate immune response. Fish Shellfish Immunol 35(3):979–987
Fijan NN (1968) Progress report on acute mortality of channel catfish fingerlings caused by a virus. Bull Off Int Epizoot 69(7):1167–1168
Fijan N, Sulimanović D, Bearzotti M, Muzinić D, Zwillenberg LO, Chilmonczyk S, Vautherot JF, de Kinkelin P (1983) Some properties of the Epithelioma papulosum cyprini (EPC) cell line from carp Cyprinus carpio. Ann Inst Pasteur Virol 134(2):207–220
Ford L, Subramaniam K, Waltzek TB, Bowser PR, Hanson L (2021) Cytochrome oxidase gene sequencing reveals channel catfish ovary cell line is contaminated with brown bullhead cells. J Fish Dis 44:119–122
Friedenreich H, Schartl M (1990) Transient expression directed by homologous and heterologous promoter and enhancer sequences in fish cells. Nucleic Acids Res 18:3299–3305
Fryer JL, Lannan CN (1996) Rickettsial infections of fish. Annu Rev Fish Dis 6:3–13
Ganassin RC, Bols NC (1998) Development of a monocyte/macrophage-like cell line, RTS11, from rainbow trout spleen. Fish Shellfish Immunol 8(6):457–476
Ghioni C, Tocher DR, Bell MV, Dick JR, Sargent JR (1999) Low C18 to C20 fatty acid elongase activity and limited conversion of stearidonic acid, 18:4(n-3), to eicosapentaenoic acid, 20:5(n-3), in a cell line from the turbot, Scophthalmus maximus. Biochim Biophys Acta 1437:170–181
Ghosh C, Collodi P (1994) Culture of cells from zebrafish (Brachydanio rerio) blastula-stage embryos. Cytotechnology 14(1):21–26
Gratacap RL, Regan T, Dehler CE (2020) Efficient CRISPR/Cas9 genome editing in a salmonid fish cell line using a lentivirus delivery system. BMC Biotechnol 20:35
Grist E, Woodhead AD, Carlson C (1986) Established cell lines from nonmammalian vertebrates: models for DNA repair studies. In Vitro Cell Dev Biol 22:677–680
Grondel JL, Gloudemans AG, van Muiswinkel WB (1985) The influence of antibiotics on the immune system. II. Modulation of fish leukocyte responses in culture. Vet Immunol Immunopathol 9(3):251–260
Hanif A, Bakopoulos V, Leonardos I, Dimitriadis GJ (2005) The effect of sea bream (Sparus aurata) broodstock and larval vaccination on the susceptibility by Photobacterium damsela subsp. piscicida and on the humoral immune parameters. Fish Shellfish Immunol 19(4):345–361
Hanson L, Dishon A, Kotler M (2011) Herpesviruses that infect fish. Viruses 3(11):2160–2191
Hao K, Yuan S, Yu F, Chen XH, Bian WJ, Feng YH, Zhao Z (2021) Acyclovir inhibits channel catfish virus replication and protects channel catfish ovary cells from apoptosis. Virus Res 292:198249
Hayasaka K, Sato M, Mitani H, Shima A (1989) Transfection of cultured fish cells RBCF-1 with exogenous oncogene and their resistance to malignant transformation. Comp Biochem Physiol 96(2):349–354
Hayasaka K, Sato M, Mitani H, Shima A (1990) Transfection of cultured fish cells RBCF-1 with exogenous oncogene and their resistance to malignant transformation. Comp Biochem Physiol B 96(2):349–354
Hayman JR, Lobb CJ (1993) Immunoglobulin in the eggs of the channel catfish (Ictalurus punctatus). Dev Comp Immunol 17(3):241–248
Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P (1995) Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol 31(1):14–24
Hedrick RP, McDowell T (1987) Passive transfer of sera with antivirus neutralizing activity from adult channel catfish protects juveniles from channel catfish virus. Trans Am Fish Soc 116:277–281
Hedrick R, Gilad O, Yun S, Spangenberg J, Marty G, Nordhausen R, Kebus M, Bercovier H, Eldar A (2000) A herpesvirus associated with mass mortality of Juvenile and Adult Koi, a strain of common carp. J Aquat Anim Health 12:44–57
Heilmann S, Ratnakumar K, Langdon E, Kansler E, Kim I, Campbell NR, Perry E, McMahon A, Kaufman C, van Rooijen E, Lee W, Iacobuzio-Donahue C, Hynes R, Zon L, Xavier J, White RM (2015) A quantitative system for studying metastasis using transparent zebrafish. Cancer Res 75(20):4272–4282
Hellberg T, Paßvogel L, Schulz KS, Klupp BG, Mettenleiter TC (2016) Nuclear egress of herpesviruses: the prototypic vesicular nucleocytoplasmic transport. Adv Virus Res 94:81–140
Helmrich A, Bailey G, Barnes DW (1988) Transfection of cultured fish cells with exogenous DNA. Cytotechnology 1:215–222
Hetrick FM, Hedrick RP (1993) New viruses described in finfish from 1988–1992. Annu Rev Fish Dis 3:187–207
Higaki S, Koyama Y, Shimada M, Ono Y, Tooyama I, Fujioka Y, Sakai N, Ikeuchi T, Takada T (2013) Response to fish specific reproductive hormones and endocrine-disrupting chemicals of a Sertoli cell line expressing endogenous receptors from an endemic cyprinid Gnathopogon caerulescens. Gen Comp Endocrinol 191:65–73
Hightower LE, Renfro JL (1988) Recent applications of fish cell culture to biomedical research. J Exp Zool 248:290–302
Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA (2010) Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 148(1):3–15
Hong Y, Winkler C, Schart M (1996) Pluripotency and differentiation of embryonic stem cell lines from the medaka fish (Oryzias latipes). Mech Dev 60(1):33–44
Hong Y, Liu T, Zhao H, Xu H, Wang W, Liu R, Chen T, Deng J, Gui J (2004) Establishment of a normal medaka fish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci U S A 101(21):8011–8016
Hong N, Li Z, Hong Y (2011) Fish stem cell cultures. Int J Biol Sci 7(4):392–402
Horbach SPJM, Halffman W (2017) The ghosts of HeLa: how cell line misidentification contaminates the scientific literature. PLoS One 12(10):e0186281
Howe K, Clark M, Torroja C (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503
Hsiao AY, Torisawa YS, Tung YC, Sud S, Taichman RS, Pienta KJ (2009) Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 30(16):3020–3027
Hsiung GD (1984) Diagnostic virology: from animals to automation. Yale J Biol Med 57:727–733
Huang YC, Han YS (2010) Anti-nervous necrosis virus drug screening using fish cell line. Conference: The American Society for Virology 29th annual meeting at: Bozeman, MT, USA, July 2010
Hughes P, Marshall D, Reid Y, Parkes H, Gelber C (2007) The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? BioTechniques 43(5):575–581
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JRJ, Joung JK (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227–229
Isa K, Shima A (1987) Transfection and stable expression of a dominant selective marker Ecogpt in a cultured cell line of the fish, Carassius auratus. J Cell Sci 88:219–224
Jabbour RE, Snyder AP (2014) Mass spectrometry-based proteomics techniques for biological identification. Biological Identification: DNA Amplification and Sequencing, Optical Sensing, Lab-On-chip and Portable Systems 1(14):370–430
Jacobson M, Gase RM (1965) Selection of appropriate tectal connections by regenerating optic fibers in adult goldfish. Exp Neurol 13(4):418–430
Jensen I, Larsen R, Robertsen B (2002) An antiviral state induced in Chinook salmon embryo cells (CHSE-214) by transfection with the double-stranded RNA poly I:C. Fish Shellfish Immunol 13:367–378
Kaur G, Dufour JM (2012) Cell lines: valuable tools or useless artifacts. Spermatogenesis 2(1):1–5
Kelly RK, Souter BW, Miller HR (1978) Fish cell lines: comparison of CHSE-214, FAIM., and RAG-2 in assaying IHN and IPN viruses. J Fish Res Board Can 35:1009–1011
Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT (2007) The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 1(1):84–96
Kienzler A, Bony S, Tronchere X, Devaux A (2013) Assessment of base-excision repair activity in fish cell lines: toward a new biomarker of exposure to environmental contaminants? Mut Res 753(2):107–113
Kim JH, Ogawa K, Wakabayashi H (2000) Lectin-reactive components of the microsporidian Glugea plecoglossi and their relation to spore phagocytosis by head kidney macrophages of ayu Plecoglossus altivelis. Dis Aquat Org 39(1):59–63
Klingelfus T, Disner GR, Voigt CL, Alle LF, Cestari MM, Leme DM (2019) Nanomaterials induce DNA-protein crosslink and DNA oxidation: a mechanistic study with RTG-2 fish cell line and comet assay modifications. Chemosphere 215:703–709
Knudsen FR, Schou AE, Wiborg ML, Mona E, Tollefsen K, Stenersen J, Sumpter JP (1997) Increase of plasma vitellogenin concentration in rainbow trout (Oncorhynchus mykiss) exposed to effluents from oil refinery treatment works and municipal sewage. Bull Environ Contam Toxicol 59(5):802–806. https://doi.org/10.1007/s001289900552
Kou GH, Wang CH, Hung HW, Jang YS, Chou CM, Lo CF (1995) A cell line (EP-1 cell line) derived from “Beko disease” affected Japanese eel elver (Anguilla japonica) persistently infected with Pleistophora anguillarum. Aquaculture 132:161–173
Krishnan K, Khanna VG, Hameed S (2010) Antiviral activity of dasyscyphin C extracted from Eclipta prostrata against fish nodavirus. J Antivirals Antiretrovirals 1:029–032
Ku CC, Teng YC, Wang CS, Lu CH (2009) Establishment and characterization of three cell lines derived from the rockfish grouper Epinephelus quoyanus: use for transgenic studies and cytotoxicity testing. Aquaculture 294:147–151
Lakra WS, Swaminathan TR, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37(1):1–20
Lannan CN (1994) Fish cell culture: a protocol for quality control. J Tissue Cult Methods 16:95–98
Lee LEJ, Clemons JH, Bechtel DG, Caldwell SJ, Han KB, Pasitschniak-Arts M, Mosser DD, Bols NC (1993) Development and characterization of a rainbow trout liver cell line expressing cytochrome P450-dependent monooxygenase activity. Cell Biol Toxicol 9:279–294
Leibovitz A (1963) The growth and maintenance of tissue-cell cultures in free gas exchange with the atmosphere. Am J Hyg 78:173–180
Leland DS, Ginocchio CC (2007) Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev 20:49–78
Leme DM (2019) Nanomaterials induce DNA-protein crosslink and DNA oxidation: a mechanistic study with RTG-2 fish cell line and comet assay modifications. Chemosphere 215:703–709
Lescat L, Véron V, Mourot B, Péron S, Chenais N, Dias K, Riera-Heredia N, Beaumatin F, Pinel K, Priault M, Panserat S, Salin B, Guiguen Y, Bobe J, Herpin A, Seiliez I (2020) Chaperone-mediated autophagy in the light of evolution: insight from fish. Mol Biol Evol 37(10):2887–2899
Liu Q, Yuan Y, Zhu F, Hong Y, Ge R (2018) Efficient genome editing using CRISPR/Cas9 ribonucleoprotein approach in cultured Medaka fish cells. Biol Open 7(8):bio035170. https://doi.org/10.1242/bio.035170
Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC (2010) Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31(32):8494–8506
Loveland PM, Wilcox JS, Pawlowski NE, Bailey GS (1987) Metabolism and DNA binding of aflatoxicol and aflatoxin B1 in vivo and in isolated hepatocytes from rainbow trout (Salmo gairdneri). Carcinogenesis 8:1065–1070
Lovitt CJ, Shelper TB, Avery VM (2014) Advanced cell culture techniques for cancer drug discovery. Biology 3(2):345–367
Luginbuhl RE, Black FL (1961) Applications of primary cell cultures in the study of animal viruses: I. The isolation and characterization of bovine and avian enteric viruses. Yale J Biol Med 33(5):339–349
Lynn DE (2009) Cell culture. In: Encyclopedia of insects, 2nd edn, pp 144–145
Ma J, Fan Y, Zhou Y, Liu W, Jiang N, Zhang J, Zeng L (2018) Efficient resistance to grass carp reovirus infection in JAM-A knockout cells using CRISPR/Cas9. Fish Shellfish Immunol 76:206–215
Ma J, Bruce TJ, Jones EM, Cain KD (2019) A review of fish vaccine development strategies: conventional methods and modern biotechnological approaches. Microorganisms 7(11):569
MacLeod RA, Dirks WG, Matsuo Y, Kaufmann M, Milch H, Drexler HG (1999) Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int J Cancer 83(4):555–563
Maguire G (2016) Therapeutics from adult stem cells and the hype curve. ACS Med Chem Lett 7(5):441–443
Mano Y, Kator K, Egami N (1982) Photoreactivation and excision repair of thymine dimers in ultraviolet irradiated cultured fish cells. J Radiat Res 90:731–737
Marecki JC, Aarattuthodiyil S, Byrd AK, Penthala NR, Crooks PA, Kevin D, Raney KD (2019) N-Naphthoyl-substituted indole thio-barbituric acid analogs inhibit the helicase activity of the hepatitis C virus NS3. Bioorg Med Chem Lett 29(3):430–434
Masters J (2002) False cell lines: the problem and a solution. Cytotechnology 39:69–74
Masters JRW, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, Toji LH, Ohno T, Tanabe H, Arlett CF, Kelland LR, Harrison M, Virmani A, Ward TH, Ayres KL, Debenham PG (2001) Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A 98:8012–8017
Matsumoto J, Lynch TJ, Grabowski S, Richards CM, Lo SJ, Clark C, Kern D, Taylor JD, Tchen IT (1983) Fish tumor pigment cells: differentiation and comparison to their normal counterparts. Am Zool 23(3):569–580
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:125–142
McIntosh D, Flaño E, Grayson TH, Gilpin ML, Austin B, Villena AJ (1997) Production of putative virulence factors by Renibacterium salmoninarum grown in cell culture. Microbiology 143:3349–3356
McKeehan W, Barnes D, Reid L, Stanbridge E, Murakami H, Sato G (1990) Frontiers in mammalian cell culture. In Vitro Cell Dev Biol 26(1):9–23
Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S (2012) Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 164:192–204
Merten OW (2006) Introduction to animal cell culture technology—past, present and future. Cytotechnology 50:1–7
Meyers TR (1979) A reo-like virus isolated from the juvenile American oyster (Crassostrea virginica). J Gen Virol 43:203–212
Miller RK (2020) A 2020 synopsis of the cell-cultured animal industry. Anim Front 10(4):64–72
Miller NW, Rycyzyn MA, Wilson MR, Warr GW, Naftel JP, Clem LW (1994) Development and characterization of channel catfish long term B cell lines. J Immunol 152:2180–2189
Miserocchi G, Mercatali L, Liverani C (2017) Management and potentialities of primary cancer cultures in preclinical and translational studies. J Transl Med 15:229
Mitani H (1984) Comparison of the radiosensitivity between three cultured fish cell lines using shortterm endpoints. Int J Radiat Biol 45:637–643
Mitani H (1986) Radiosensitivity of primary cultured fish cells with different ploidy. J Radiat Res 27:284–290
Mitani H, Egami N (1980) Long-term cultivation of the medaka (Oryzias latipes) cells from liver tumors induced by diethylnitrosamine. J Fac ScL Univ Tokyo Sec IV 14:391–398
Mitani H, Egami N (1982) Rejoining of DNA strand breaks after gamma-irradiation in cultured fish cells, CAF-MM1. Int J Radiat Biol 41:85–90
Mitani H, Naruse K, Shima A (1989) Eurythermic and stenothermic growth of cultured fish cells and their thermosensitivity. J Cell Sci 93:731–737
Mitani H, Komura JI, Shima A (1990) The repair of UV-irradiated plasmids transfected into cultured fish cells. Mut Res 236:77–84
Miyazaki T, Goto K, Kobayashi T, Kageyama T, Miyata M (1999) Mass mortalities associated with a virus disease in Japanese pearl oyster Pinctada fucata martensii. Dis Aquat Org 37:1–12
Mor A, Avtalion RR (1990) Transfer of antibody-activity from immunized mother to embryo in tilapias. J Fish Biol 37(2):249–255
Mori S, Sakakura E, Tsunekawa Y, Hagiwara M, Suzuki T, Eirakuet M (2019) Self-organized formation of developing appendages from murine pluripotent stem cells. Nat Commun 10:3802
Morin G, Pinel K, Dias K, Seiliez I, Beaumatin F (2020) RTH-149 cell line, a useful tool to decipher molecular mechanisms related to fish nutrition. Cell 9(8):1754
Nakada N, Nyunoya H, Nakamura M, Hara A, Iguchi T, Takada H (2004) Identification of estrogenic compounds in wastewater effluent. Environ Toxicol Chem 23:2807–2815
Nelson-Rees WA, Daniels DW, Flandermeyer RR (1981) Cross-contamination of cells in culture. Science 212(4493):446–452
Nicholson BL (1989) Fish cell culture: an update. Adv Cell Cult 7:1–18
Nielsen CV, Buchmann K (2000) Prolonged in vitro cultivation of Ichthyophthirius multifiliis using an EPC cell line as substrate. Dis Aquat Org 42:215–219
Noga EJ, Hartmann JX (1981) Establishment of walking catfish (Clarius batrachus) cell lines and development of a channel catfish (Ictalurus punctatus) virus vaccine. Can J Fish Aquat Sci 38:925–930
Novaro V, Roskelley CD, Bissell MJ (2003) Collagen-IV and laminin-1 regulate estrogen receptor α expression and function in mouse mammary epithelial cells. J Cell Sci 116(14):2975–2986
Nusbaum KE, Smith BF, DeInnocentes P, Bird RC (2002) Protective immunity induced by DNA vaccination of channel catfish with early and late transcripts of the channel catfish herpesvirus. Vet Immunol Immunopathol 84:151–168
Officer JE (1964) The ability of a fish cell line to support the growth of mammalian viruses. Proc Soc Exp Biol Med 116:190–194
Olsson PE, Hyllner SJ, Zafarullah M, Andersson T, Gedamu L (1990) Differences in metallothionein gene expression in primary cultures of rainbow trout hepatocytes and the RTH-149 cell line. Biochim Biophys Acta 1049(1):78–82
Olsen AB, Melby HP, Speilberg L, Evensen Ø, Hastein T (1997) Piscirickettsia salmonis infection in Atlantic salmon Salmo salar in Norway-epidemiological, pathological and microbiological findings. Dis Aquat Org 31:35–48
Ong SM, Zhao Z, Arooz T, Zhao D, Zhang S, Du T (2010) Engineering a scaffold-free 3D tumor model for in vitro drug penetration studies. Biomaterials 31(6):1180–1190
Ortiz E, Gurrola GB, Schwartz EF, Possani LD (2015) Scorpion venom components as potential candidates for drug development. Toxinology 93:125–135
Ott T (2004) Tissue culture of fish cell lines. The National Wild Fish Health Survey (NWFHS) Laboratory Procedure Manual 2(10):1–16
Pampaloni F, Reynaud EG, Stelzer EHK (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8(10):839–845
Pan C, Kumar C, Bohl S, Klingmueller U, Mann M (2009) Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol Cell Proteomics 8(3):443–450
Pandey G (2013) Overview of fish cell lines and their uses. Int J Pharm Res Sci 2(3):580–590
Pandey S, Upadhyay RK (2020) The fish venom toxins: natural source of pharmaceuticals and therapeutic agents “pharmaceutical and therapeutic uses of fish venom toxins”. Int J Pharm Pharm Sci 12(11):1–14
Parameswaran P, Ahmed VP, Shukla R, Bhonde RR, Sahul Hameed AS (2007) Development and characterization of two new cell lines from milkfish (Chanos chanos) and grouper (Epinephelus coioides) for virus isolation. Mar Biotechnol 9:281–291
Park EH, Lee JS, Yi AE, Etoh H (1989) Fish cell line (ULF-23HU) derived from the fin of the central mudminnow (Umbra limi): suitable characteristics for clastogenicity assay. In Vitro Cell Dev Biol 25:987–994
Paw BH, Zon LI (1999) Primary fibroblast cell culture. Methods Cell Biol 59:39–43
Pelissero C, Flouriot G, Foucher JL, Bennetau B, Dunogues J, Le GF, Sumpter JP (1993) Vitellogenin synthesis in cultured hepatocytes; an in vitro test for the estrogenic potency of chemicals. J Steroid Biochem Mol Biol 44:263–272
Peng CA, Wang CH, Wang WL (2010) Rapid antiviral assay using QD-tagged fish virus as imaging nanoprobe. J Virol Methods 169(2):412–415
Perelberg A, Ronen A, Hutoran M, Smith Y, Kotler M (2005) Protection of cultured Cyprinus carpio against a lethal viral disease by an attenuated virus vaccine. Vaccine 23:3396–3403
Pham AK, Durocher Y (2006) Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol Biotechnol 34:225–237
Pickl M, Ries CH (2008) Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 28(3):461–468
Plumb JA (1986) Channel catfish virus disease. US fish and wildlife publications. Fish Dis Leaflet 73:1–7
Potter G, Smith AST, Vo NTK, Muster J, Weston W, Bertero A, Maves L, Mack DL, Rostain A (2020) A more open approach is needed to develop cell-based fish technology: it starts with zebrafish. One Earth 3(1):54–64
Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP (1994) Estrogenic effects of effluents from sewage treatment works. Chem Ecol 8:275–285
Puschhof J, Post Y, Beumer J, Kerkkamp HM, Bittenbinder M, Vonk FJ, Casewell NR, Richardson MK, Clevers H (2021) Derivation of snake venom gland organoids for in vitro venom production. Nat Protoc 16(3):1494–1510. https://doi.org/10.1038/s41596-020-00463-4
Qin QW, Wu TH, Jia TL, Hegde A, Zhang RQ (2006) Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus. J Virol Methods 131:58–64
Quiot JM, Vey A, Vago C (1985) Effect of mycotoxins on invertebrate cells in vitro. Adv Cell Cult 4:199–212
Rachlin JW, Perlmutter A (1968) Fish cells in culture for study of aquatic toxicants. Water Res 2(6):409–414
Rapanan JL, Pascual AS, Uppalapati CK, Cooper KE, Leyva KJ, Hull EE (2015) Zebrafish keratocyte explants to study collective cell migration and reepithelialization in cutaneous wound healing. J Vis Exp 96:52489
Rausch DM, Simpson SB (1988) In vivo test system for tumor production by cell lines derived from lower vertebrates. In Vitro Cell Dev Biol 24:217–222
Regan JD, Cook JS (1967) Photoreactivation in an established vertebrate cell line. Proc Natl Acad Sci U S A 58:2274–2279
Rehberger K, Kropf C, Segner H (2018) In vitro or not in vitro: a short journey through a long history. Environ Sci Eur 30:23
Reichert M, Bergmann SM, Hwang J, Buchholz R, Lindenberger C (2017) Antiviral activity of exopolysaccharides from Arthrospira platensis against koi herpesvirus. J Fish Dis 40:1441–1450
Rivers TM (1937) Viruses and Koch’s postulates. J Bacteriol 33(1):1–12
Ronen A, Perelberg A, Abramowitz J, Hutoran M, Tinman S, Bejerano I, Steinitz M, Kotler M (2003) Efficient vaccine against the virus causing a lethal disease in cultured Cyprinus carpio. Vaccine 21:4677–4684
Rubio N, Datar I, Stachura D, Kaplan D, Krueger K (2019) Cell-based fish: a novel approach to seafood production and an opportunity for cellular agriculture. Front Sustain Food Syst 3:43. https://doi.org/10.3389/fsufs.2019.00043
Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen, London
Rutishauser BV, Pesonen M, Escher BI, Ackermann GE, Aerni HR, Suter MJF, Eggen RIL (2004) Comparative analysis of estrogenic activity in sewage treatment plant effluents involving three in vitro assays and chemical analysis of steroids. Environ Toxicol Chem 23:857–864
Ryan LA, Seymour CB, O’Neill-Mehlenbacher A, Mothersill CE (2008) Radiation-induced adaptive response in fish cell lines. J Environ Radioact 99(4):739–747
Ryan LA, Seymour CB, Joiner MC, Mothersill CE (2009) Radiation-induced adaptive response is not seen in cell lines showing a bystander effect but is seen in lines showing HRS/IRR response. Int J Radiat Biol 85:87–95
Saggers BA, Gould ML (1989) The attachment of microorganisms to macrophages isolated from the tilapia Oreochromis spilurus (Gunther). J Fish Biol 35(2):287–294
Santini MT, Rainaldi G, Indovina PL (1999) Multicellular tumour spheroids in radiation biology. Int J Radiat Biol 75(7):787–799
Schippers IJ, Moshage H, Roelofsen H, Müller M, Heymans HS, Ruiters M, Kuipers F (1997) Immortalized human hepatocytes as a tool for the study of hepatocytic (de-)differentiation. Cell Biol Toxicol 13:375–386
Schirmer K (2006) Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicology 224(3):163–183
Schultz E, Jaryszak DL, Gibson MC, Albright DJ (1986) Absence of exogenous satellite cell contribution to regeneration of frozen skeletal muscle. J Muscle Res Cell Motil 7:361–367
Segner H (1998) Fish cell lines as a tool in aquatic toxicology. Experientia Suppl 86:1–38
Segner H, Braunbeck T (2003) End points for in vitro toxicity testing with fish cells. In: Austin B, Mothersill C (eds) In vitro methods in aquatic toxicology. Springer Praxis, Chichester
Shaw RW, Kent ML, Adamson ML (2001) Phagocytosis of Loma salmonae (Microsporidia) spores in Atlantic salmon (Salmo salar), a resistant host and Chinook salmon (Oncorhynchus tshawytscha), a susceptible host. Fish Shellfish Immunol 11:91–100
Shield K, Ackland ML, Ahmed N, Rice GE (2009) Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol 113(1):143–148
Shima A, Setlow RB (1985) Establishment of a cell line (PF line) from a gynogenetic teleost, Poecilia formosa (Girard), and characterization of its repair ability of UV-induced DNA damage. Zool Sci 2:477–483
Shima A, Nikaido O, Shinohara S, Egami N (1980) Continued in vitro growth of fibroblast-like cells (RBCF-1) derived from the caudal fin of the fish Carassius auratus. Exp Gerontol 15:305–314
Shima A, Ikenaga M, Nikaido O, Takebe H, Egami N (1981) Photoreactivation of ultraviolet lightinduced damage in cultured fish cells as revealed by increased colony-forming ability and decreased content of pyrimidine dimers. Photochem Photobiol 33:313–316
Shima A, Isa K, Komura J, Hayasaka K, Mitani H (1987) Somatic cell genetic study of DNA repair in cultured fish cells. In: Kuroda Y, Kurstak E, Maramorosch K (eds) invertebrate and fish tissue culture. Japan Scientific Societies Press, Tokyo, pp 215–217
Smeets JMW, Rankouhi TR, Nichols KM, Komen H, Kaminski NE, Giesy JP, van den Berg M (1999) In vitro vitellogenin production by carp (Cyprinus carpio) hepatocytes as a screening method for determining (anti)estrogenic activity of xenobiotics. Toxicol Appl Pharmacol 157(1):68–76
Sommerset I, Krossøy B, Biering E, Frost P (2005) Vaccines for fish in aquaculture. Expert Rev Vaccines 4:89–101
Stinehardt E, Israeli C, Lambert R (1913) Studies on the cultivation of the virus of vaccinia. J Infect Dis 13:204–300
Sumpter JP, Jobling S (1995) Vitellogenesis as a biomarker for estrogenic contamination in the aquatic environment. Environ Health Perspect 103:173–178
Sung KE, Su X, Berthier E, Pehlke C, Friedl A, Beebe DJ (2013) Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One 8(10):e76373
Sykes JE, Rankin SC (2014) Isolation in cell culture. Canine Feline Infect Dis 1:2–9
Taju G, Abdul Majeed S, Nambi KSN, Sahul Hameed AS (2017) Application of fish cell lines for evaluating the chromium induced cytotoxicity, genotoxicity and oxidative stress. Chemosphere 184:1–12
Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, Haist-Gulde B, Preuss G, Wilme U, Zulei-Seibert N (2002) Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 36(17):3855–3863
Thangaraj RS, Ravi C, Kumar R, Dharmaratnam A, Saidmuhammed BV, Pradhan PK, Sood N (2018) Derivation of two tilapia (Oreochromis niloticus) cell lines for efficient propagation of tilapia lake virus (TiLV). Aquaculture 492:206–214
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Thorpe KL, Maack G, Benstead R, Tyler CR (2009) Estrogenic wastewater treatment works effluents reduce egg production in fish. Environ Sci Technol 43(8):2976–2982
Till JE, McCulloch EA (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14:213–222
Troszok A, Kolek L, Szczygieł J, Wawrzeczko J, Borzym E, Reichert M, Kamińska T, Ostrowski T, Jurecka P, Adamek M, Rakus K, Irnazarow I (2018) Acyclovir inhibits cyprinid herpesvirus 3 multiplication in vitro. J Fish Dis 41(11):1709–1718
Tsugawa K, Lagerspetz K (1990) Direct adaptations of cells to temperature: membrane fluidity of goldfish cells cultured in vitro at different temperatures. Comp Biochem Physiol 96A(1990):57–60
Tsuruga Y, Kiyono T, Matsushita M, Takahashi T, Kasai H, Todo S (2008) Establishment of immortalized human hepatocytes by introduction of HPV16 E6/E7 and hTERT as cell sources for liver cell-based therapy. Cell Transplant 17:1083–1094
Ulrich AB, Pour PM (2001) Cell lines. In: Brenner S, Miller JH (eds) Encyclopedia of genetics. Academic Press, Cambridge, MA
Uysal O, Sevimli T, Sevimli M, Gunes S, Sariboyaci AE (2018) Cell and tissue culture: the base of biotechnology. Omics technologies and bio-engineering, pp 391–429
Vallejo AN, Ellsaesser CF, Miller NW, Clem LW (1991) Spontaneous development of functionally active long-term monocyte-like cell lines from channel catfish. In Vitro Cell Dev Biol 27(4):279–286
Van Der Ven PFM, Fürst DO (1998) Expression of sarcomeric proteins and assembly of myofibrils in the putative myofibroblast cell line BHK-21/C13. J Muscle Res Cell Motil 19:767–775
Verma D, Kim EA, Swaminathan S (2013) Cell-based screening assay for antiviral compounds targeting the ability of herpesvirus posttranscriptional regulatory proteins to stabilize viral mRNAs. J Virol 87(19):10742–10751
Verma A, Verma M, Singh A (2020) Animal tissue culture principles and applications. Anim Biotechnol:269–293
Vertrees R, Goodwin T, Jordan JM, Zwischenberger JB (2008) Tissue culture models. In: Zander DS, Popper HH, Jagirdar J, Haque AK, Cagle PT, Barrios R (eds) Molecular pathology of lung diseases. Molecular pathology library. Springer, New York, pp 150–165
Villena AJ (2003) Applications and needs of fish and shellfish cell culture for disease control in aquaculture. Rev Fish Biol Fish 13:111–140
Wagle M, Jesuthasan S (2003) Baculovirus-mediated gene expression in zebrafish. Mar Biotechnol 5:58–63
Waltzek TB, Kelley GO, Alfaro ME, Kurobe T, Davison AJ, Hedrick RP (2009) Phylogenetic relationships in the family Herpesviridae. Dis Aquat Org 84:179–194
Wang Q, Fang J, Pan Q, Wang Y, Xue T, Li L, Chen T (2018) Efficient and stable delivery of multiple genes to fish cells by a modified recombinant baculovirus system. Int J Mol Sci 19(12):3767
Weiswald LB, Bellet D, Dangles-Marie V (2015) Spherical cancer models in tumor biology. Neoplasia 17(1):1–15
Wise DJ, Greenway TE, Byars TS, Griffin MJ, Khoo LH (2015) Oral vaccination of channel catfish against enteric septicemia of catfish (ESC) using a live attenuated Edwardsiella ictaluri vaccine. J Aquat Anim Health 27:135–143
Wolf K (1988) Fish viruses and fish viral diseases. Cornell University Press, Ithaca, NY
Wolf K, Ahne W (1982) Fish cell culture. Adv Cell Cult 2:305–327
Wolf K, Darlington RW (1971) Channel catfish virus: a new herpesvirus of ictalurid fish. J Virol 8(4):525–533
Wolf K, Quimby MC (1962) Established eurythermic line of fish cells in vitro. Science 135(3508):1065–1066
Wolf K, Quimby MC (1969) Fish cell and tissue culture. Fish Physiol 3:253–305
Wolf K, Quimby MC (1976a) Primary monolayer culture of fish cells initiated from minced tissues. Tissue Cult Assoc Manual 2:445–448
Wolf K, Quimby MC (1976b) Primary monolayer culture of fish cells initiated from trypsinized tissues. Tissue Cult Assoc Manual 2:453–456
Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J (2009) Collagen-based cell migration models in vitro and in vivo. Cell Dev Biol 20(8):931–941
Wongtavatchai J, Conrad PA, Hedrick RP (1994) In vitro cultivation of the microsporidian: enterocytozoon salmonis using a newly developed medium for salmonid lymphocytes. J Tissue Cult Methods 16:125–131
Xu Z, Gao Y, Hao Y, Li E, Wang Y, Zhang J (2013) Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 34(16):4109–4117
Yamada KM, Cukierman E (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130(4):601–610
Yan Y, Du J, Chen T, Yi M, Li M, Wang S, Li CM, Hong Y (2009) Establishment of medaka fish as a model for stem cell-based gene therapy: efficient gene delivery and potential chromosomal integration by baculoviral vectors. Exp Cell Res 315:2322–2331
Yang J, Liu A, Dougherty C, Chen X, Guzman R, Nandi S (2000) Estrogen and progesterone receptors can be maintained in normal human breast epithelial cells in primary culture and after transplantation into nude mice. Oncol Rep 7(1):17–21
Ye HQ, Chen SL, Sha ZX, Xu MY (2006) Development and characterization of cell lines from heart, liver, spleen and head kidney of sea perch Lateolabrax japonicus. J Fish Biol 69:115–126
Yi M, Hong N, Hong Y (2009) Generation of medaka fish haploid embryonic stem cells. Science 326:430–433
Yip JH, Bols NC (1982) The fusion of trout spermatozoa with Chinese hamster fibroblasts. J Cell Sci 53:307–321
Yoshizaki G, Ichikawa M, Hayashi M, Iwasaki Y, Miwa M, Shikina S, Okutsu T (2010) Sexual plasticity of ovarian germ cells in rainbow trout. Dev Stem Cells 137:1227–1230
Zafarullah M, Bonham K, Gedamu L (1988) Structure of the rainbow trout metallothionein B gene and characterization of its metal-responsive region. Mol Cell Biol:84469–84476
Zafarullah M, Olsson PE, Gedamu L (1989) Endogenous and heavy metal ion induced metallothionein gene expression in salmonid fish tissues and cell lines. Gene 83:85–93.286
Zeeman MG, Brindley WA (1981) Effects of toxic agents upon fish immune system: a review. In: Sharma RP (ed) Immunological considerations in toxicology, vol 2. CRC Press, Boca Raton, pp 1–60
Zegura B, Filipic M (2019) The application of the comet assay in fish cell lines. Mut Res 842:72–84
Zhang Q, Cooper RK, Wolters WR, Tiersch TR (1998) Isolation, culture and characterization of a primary fibroblast cell line from channel catfish. Cytotechnology 26:83–90
Zhang CL, Huang T, Wu BL, He WX, Liu D (2017) Stem cells in cancer therapy: opportunities and challenges. Oncotarget 8(43):75756–75766
Zhou GZ, Li ZQ, Yuan XP, Zhang QY (2007) Establishment, characterization, and virus susceptibility of a new marine cell line from red spotted grouper (Epinephelus akaara). Mar Biotechnol 9:370–376
Acknowledgments
The authors acknowledge financial support from the Mississippi Agricultural and Forestry Experiment Station (MAFES) Strategic Research Initiative and the US Department of Agriculture—Agricultural Research Service—Catfish Health Initiative.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Aarattuthodi, S., Dharan, V. (2021). Applications of Fish Cell Cultures. In: Gupta, S.K., Giri, S.S. (eds) Biotechnological Advances in Aquaculture Health Management . Springer, Singapore. https://doi.org/10.1007/978-981-16-5195-3_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-5195-3_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5194-6
Online ISBN: 978-981-16-5195-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)