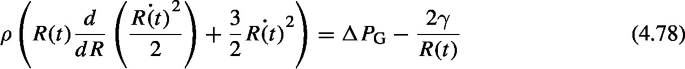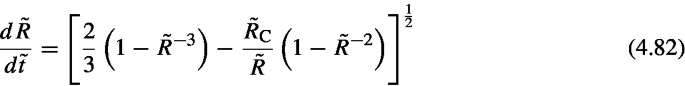Abstract
Bubbles nucleating in supersaturated liquids grow by \({\text {H}_{2}}\text {O}\) diffusion in melts into bubbles (the number of \({\text {H}_{2}}\text {O}\) molecules in the bubbles increases) and expand by decompression (the number of \({\text {H}_{2}}\text {O}\) molecules in the bubbles does not change but the molecular volume increases). In this manner, “growth” (“grow”) and “expansion” (“expand”) are used differently for different phenomena in this chapter. Although growth and expansion simultaneously progress, their behavior will be separately understood by ignoring the other at first. Then, the two phenomena will be combined to understand the experimental results. In this chapter, important factors including time constants of various elemental processes and their dimensionless number ratios, such as the Pélclet number, which is the ratio of the diffusion to the viscosity-limited expansion rate, are discussed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Notes
- 1.
It slightly differs from crystal growth. Reaction-controlled crystal growth rate is driven by difference between concentration in liquid and equilibrium concentration at interface (i.e., the degree of supersaturation).
- 2.
This equation slightly differs from the equation for crystallization (Eq. 7.38). This results from (1) difference in the equation of state between bubbles and crystals (This is attributed to the equation of state of gas, whereas crystals are assumed to be linear elastic bodies) and (2) difference in activity relation (water is proportional to the square of concentration, whereas crystals are assumed to be an ideal solution). If an ideal gas \(P_\mathrm{G}^{*} = k_\mathrm{B} T / v_\mathrm{G} \) is applied to gas, the equation agrees with the corresponding definitional equation of crystallization in the following form: \(R_{P}^{*} = 2 \gamma v_\mathrm{G} / k_\mathrm{B} T \).
- 3.
Assuming \(y=C_\mathrm{eq}/C, y^{-2} -1\) gives an estimate of the dimensionless degree of supersaturation, \(\Delta \), and the dimensionless degree of supersaturation \(\Delta \) and the critical nucleus radius \(R_\mathrm{C}\) generally have a relationship

then

can be written.
- 4.
In actuality, \(v_\mathrm{G}\) changes in accordance with the equation of state, e.g., \(v_\mathrm{G} = k_\mathrm{B} T/ P_\mathrm{G}\).
- 5.
Difference from the case of crystallization lies at 2 in the denominator.
- 6.
For the initial delay in bubble growth, when Proussevitch et al. (1993) first indicated it in calculation of bubble growth by a cell model, Sparks (1994) argued that the delay was attributed to viscosity. Sparks (1978) had reviewed the problem of the initial delay in bubble growth and had recognized the problem of the critical size at which the surface tension was effective, as well as the influence of viscosity. Against this, Proussevitch et al. (1993) showed additional simulation results and were adamant that it was the effect of interfacial tension. After that, Navon et al. (1998) revealed that the delay in bubbles’ viscosity-limited growth has exponential characteristics.
- 7.
- 8.
Taking \(\ddot{R}=d \dot{R} / dt = d \dot{R} /dR \cdot d R/ dt = \dot{R} \cdot d \dot{R} /dR\) into consideration, Eq. (4.76) can be rewritten as
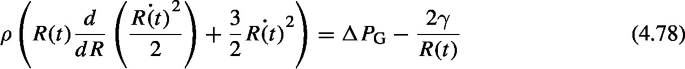
Assuming \(y= \dot{R(t)}^{2} /2\),
$$ \frac{dy}{dR} + \frac{3}{R} y = \frac{1}{R} \left( \frac{\Delta P_\mathrm{G}}{\rho } - \frac{2 \gamma }{\rho } \frac{1}{R} \right) $$is obtained, which is an inhomogeneous first-order linear differential equation with respect to y as a function of R and can be solved by the variation of constants method. Assuming \(y=0\) when \(R=R(0)=R_\mathrm{B}\), the constants of integration (0) can be determined.
- 9.
Scaling of the above equation by this timescale (\(\tilde{R}=R/R(0)\), \(\tilde{t} = t/t_\mathrm{c}\)) gives
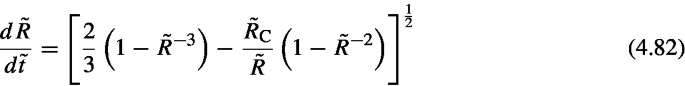
- 10.
The Péclet number defined here does not represent the ratio of advection to diffusion conventionally used in matter transport. The conventionally used ratio is the ratio of the advection term of the diffusion equation (the second term on the left-hand side of Eq. (4.1)) to the diffusion term (the right-hand side of Eq. (4.1)), such as the Péclet number described in Sect. 4.3.2. However, note that the advection term is not included in the discussion here.
- 11.
Between diffusivity and viscosity, the classical Stokes-Einstein relation holds

in some cases. In this equation, \(a_{\mathrm {M}}\) is the effective radius of a molecule; however, this relation does not hold between the viscosity and the molecular diffusion of silicate melt (Liang et al. 1997).
- 12.
You can convert the unit of concentration from wt% to (number of molecules/m\(^{3}\)) by replacing \(\rho _\mathrm{G} / \rho \)
 \(P_\mathrm{G} / k_\mathrm{B} T = 1/ v_\mathrm{G}(P_\mathrm{G})\), where \(v_\mathrm{G}(P_\mathrm{G})\) is the volume of one water molecule in gas at the pressure \(P_\mathrm{G}\).
\(P_\mathrm{G} / k_\mathrm{B} T = 1/ v_\mathrm{G}(P_\mathrm{G})\), where \(v_\mathrm{G}(P_\mathrm{G})\) is the volume of one water molecule in gas at the pressure \(P_\mathrm{G}\).
References
Amon M, Denson CD (1984) A study of the dynamics of foam growth: analysis of the growth of closely spaced spherical bubbles. Polymer Eng Sci 24:1026–1034
Barclay J, Riley DS, Sparks RSJ (1995) Analytical models for bubble growth during decompression of high viscosity magmas. Bull Volcanol 57:422–431
Bird RB, Stewart WE, Lightfoot EN (1960) Transport phenomena. Wiley, New York, p 780
Blower JD, Madera HM, Wilson SDR (2001) Coupling of viscous and diffusive controls on bubble growth during explosive volcanic eruptions. Earth Planet Sci Lett 193:47–56
Blundy J, Cashman K, Humphreys M (2006) Magma heating by decompression-driven crystallization beneath andesite volcanoes. Nature 443(7107):76
Fogler HS, Goddard JD (1970) Collapse of spherical cavities in viscoelastic fluids. Phys Fluid 13:1135–1141
Gardner JE, Hilton M, Carroll MR (1999) Experimental constraints on degassing of magma: isothermal bubble growth during continuous decompression from high pressure. Earth Planet Sci Lett 168:201–218
Gondé C, Martel C, Pichavant M, Bureau H (2011) In situ bubble vesiculation in silicic magmas. Am Mineral 96(1):111–124
Ichihara M, Okunitani H, Ida Y, Kameda M (2004) Dynamics of bubble oscillation and wave propagation in viscoelastic liquids. J Volcanol Geotherm Res 129:37–60
Keller JB, Miksis M (1980) Bubble oscillations of large amplitude. J Acoustical Soc Am 68(2):628–633
Lensky NG, Navon O, Lyakhovsky V (2004) Bubble growth during decompression of magma: experimental and theoretical investigation. J Volcanol Geotherm Res 129:7–22
Liang Y, Richter FM, Chmberlin L (1997) Diffusion in silicate melts: III. Emprical models for multicomponent diffusion. Geochim Cosmochim Acta 61:5295–5312
Liu Y, Zhang Y, Behrens H (2004) H\(_{2}\)O diffusion in dacitic melts. Chem Geol 209:327–340
Lyakhovsky V, Hurwitz S, Navon O (1996) Bubble growth in rhyolitic melts: experimental and numerical investigation. Bull Volcanol 58:19–32
Martel C, Bureau H (2001) In situ high-pressure and high-temperature bubble growth in silicic melts. Earth Planet Sci Lett 191:115–127
Navon O, Chekhmir A, Lyakhovsky V (1998) Bubble growth in highly viscous melts: Theory, experiments, and autoexplosivity of dome lavas. Earth Planet Sci Lett 160:763–776
Plesset MS, Prosperetti A (1977) Bubble dynamics and cavitation. Ann Rev Fluid Mech 9:145–185
Poritsky H (1952) The collapse or growth of a spherical bubble or cabity in a viscous fluid. Proc First Nat Cong Appl Mech 813–821-D
Proussevitch AA, Sahagian DL, Kutolin VA (1993) Stability of foams in silicate melts. J Volcanol Geotherm Res 59:161–178
Proussevitch AA, Sahagian DL, Anderson VAT (1993) Dynamics of diffusive bubble growth in magmas: isothermal case. J Geophys Res 98:22283–22307
Proussevitch AA, Sahagian DL, Anderson VAT (1994) Reply to comments by sparks. J Geophys Res 99:17829–17832
Rayleigh L (1917) On the pressure developed in a liquid during the collapse of a spherical cavity. Philosophical Mag J Sci 34:94–98
Sahagian DL, Proussevitch AA (1996) Thermal effects of magma degassing. J Volcanol Geotherm Res 74:19–38
Shaw H (1974) Diffusion of H\(_{2}\)O in granitic liquids: part I. experimental data; part II. Mass transfer in magma chambers. In: Geochemical transport and kinetics, vol 634. Carnegie I, Washington, pp 139–170
Sparks RSJ (1994) Comments on “Dynamics of diffusive bubble growth in magmas: Isothermal case” by A. A. Proussevitch, D. L. Sahagian, and V. A. T. Anderson. J Geophys Res 99:17827–17828
Sparks RSJ (1978) The dynamics of bubble formation and growth in magmas: a review and analysis. J Volcanol Geotherm Res 3:1–37
Toramaru A (1995) Numerical study of nucleation and growth of bubbles in viscous magmas. J Geophys Res 100:1913–1931
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Toramaru, A. (2022). Growth and Expansion of Bubbles. In: Vesiculation and Crystallization of Magma. Advances in Volcanology. Springer, Singapore. https://doi.org/10.1007/978-981-16-4209-8_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-4209-8_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4208-1
Online ISBN: 978-981-16-4209-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)