Abstract
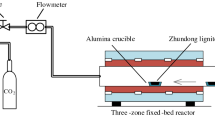
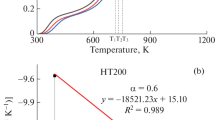
The gasification characteristics and the morphology of the residue ash from pressurised K2CO3-catalysed gasification of a Chinese lignite in CO2 was investigated using a High-Pressure Thermogravimetric Analyser operating at pressure of 2.0 or 3.5 MPa and temperature between 750 and 900 °C for at least 2 h, after being heated from room temperature at 10 °C/min. The K2CO3 addition was varied from 0 to 10% w/w. Gasification characteristics of the K2CO3-doped lignite was determined by analysing the weight loss and conversion rate as a function of time whereas the ash morphology was analysed by using SEM–EDS. Results showed that at 3.5 MPa the in-situ weight loss of the lignite increased as K2CO3 addition ratio increased, suggesting that K2CO3 addition promoted lignite gasification. The conversion rate of the lignite correspondingly increased from 61 to 92% as the temperature elevated to 750 °C. An increase in the final temperature to 900 °C significantly promoted lignite gasification when K2CO3 was less than 5%, however this was not obvious for lignite with 10% K2CO3 addition. This is because the conversion rate of the lignite with 10% K2CO3 addition had exceeded 90% before the final temperature of 900 °C was reached. Furthermore, as pressure decreased from 3.5 to 2.0 MPa, the lignite gasification rate slowed down, with or without K2CO3 addition. Conversion rate of the lignite decreased from 61 to 42% while the temperature initially elevated to 750 °C. SEM–EDS analysis revealed that sintering of the lignite ash was not observed at 750 °C, but became apparent at 1% K2CO3 addition. The degree of ash sintering further aggravated at 5 and 10% K2CO3 addition. As the temperature increased from 750 to 900 °C, the ashes of the raw lignite and 1% K2CO3 doped-lignite remained largely similar, whereas the sizes of the 10% K2CO3-doped lignite ash was increased and the particle surfaces became smooth, suggesting an enhanced sintering of the ash. The formation of K-aluminosilicate and Ca-aluminosilicate of low-melting points in the ash was responsible for possible deactivation of the doped catalyst K2CO3 and the observed ash behaviour.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Akyurtlu JF, Akyurtlu A (1995) Catalytic gasification of Pittsburgh coal char by potassium sulphate and ferrous sulphate mixtures. Fuel Process Technol 43(1):71–86
Bai J, Li W (2011) Effects of mineral matter and coal blending on gasification. Energy Fuels 25(3):1127–1131
Bruno G, Carvani L, Passoni G (1986) Correlation between potassium losses and mineral matter composition in catalytic coal steam gasification. Fuel 65(10):1473–1475
Chen J, Dai B (2012) XANES investigation on sulfur evolution during victorian brown coal char gasification in oxy-fuel combustion mode. Energy Fuels 26(8):4775–4782
Duchesne MA, Hall AD (2014) Fate of inorganic matter in entrained-flow slagging gasifiers: fuel characterization. Fuel Process Technol 118:208–217
Erickson T, Benson S (1992) Coal ash behavior in reducing environments. North Dakota University, Grand Forks, ND (United States). Energy and Environmental Research Center
Fan S, Yuan X (2016) Experimental and kinetic study of catalytic steam gasification of low rank coal with an environmentally friendly, inexpensive composite K2CO3–eggshell derived CaO catalyst. Fuel 165:397–404
Formella K, Leonhardt P (1986) Interaction of mineral matter in coal with potassium during gasification. Fuel 65(10):1470–1472
Gupta RP (2005) Research in Newcastle—past, present and future. Fuel 84(10):1176–1188
Hattingh BB, Everson RC (2011) Assessing the catalytic effect of coal ash constituents on the CO2 gasification rate of high ash, South African Coal. Fuel Process Technol 92(10):2048–2054
Irfan MF (2011) Coal gasification in CO2 atmosphere and its kinetics since 1948: a brief review. Energy 36(1):12–40
Jaffri G-e-R, Zhang J-Y (2008) Catalytic gasification characteristics of mixed black liquor and calcium catalyst in mixing (air/steam) atmosphere. J Fuel Chem Technol 36(4):406–414
Jing N, Wang Q (2011a) Effect of different reaction atmospheres on the sintering temperature of Jincheng coal ash under pressurized conditions. Fuel 90(8):2645–2651
Jing N, Wang Q (2011b) Effect of chemical composition on sintering behavior of Jingcheng coal ash under gasification atmosphere. Chem Eng Commun 199(2):189–202
Jing N, Wang Q (2013a) Effect of temperature and pressure on the mineralogical and fusion characteristics of Jincheng coal ash in simulated combustion and gasification environments. Fuel 104:647–655
Jing N, Wang Q (2013b) The sintering behavior of coal ash under pressurized conditions. Fuel 103:87–93
Jing N, Zhu M (2016) Effect of ash preparation method on the sintering characteristics of ashes from combustion of coal and biomass blends. Fuel 186:830–837
Kühn L, Plogmann H (1983) Reaction of catalysts with mineral matter during coal gasification. Fuel 62(2):205–208
Lee WJ, Kim SD (1995) Catalytic activity of alkali and transition metal salt mixtures for steam-char gasification. Fuel 74(9):1387–1393
Li F, Huang J (2010) Formation mechanism of slag during fluid-bed gasification of lignite. Energy Fuels 25(1):273–280
Li J, Zhu M (2017) Effect of coal blending and ashing temperature on ash sintering and fusion characteristics during combustion of Zhundong lignite. Fuel 195(Supplement C):131–142
Liang D, Xie Q (2018) Catalytic effect of alkali and alkaline earth metals in different occurrence modes in Zhundong coals. Asia-Pacific J Chem Eng 13(3):e2190
Liu Y, Guan Y-J (2018a) Gasification reactivity and morphology of coal chars formed in N2 and CO2 atmospheres. Chem Pap 7(8):2045–2054
Liu Y, Guan Y (2018b) CO2 gasification performance and alkali/alkaline earth metals catalytic mechanism of Zhundong coal char. Korean J Chem Eng 35(4):859–866
Nzihou A, Stanmore B (2013) A review of catalysts for the gasification of biomass char, with some reference to coal. Energy 58:305–317
Sharma A, Takanohashi T (2008) Effect of catalyst addition on gasification reactivity of HyperCoal and coal with steam at 775–700°C. Fuel 87(12):2686–2690
Takematsu T, Maude C (1991) Coal gasification for igcc power generation. IEACR/37. IEA Coal Research, London
van Dyk JC, Waanders FB (2007) Manipulation of gasification coal feed in order to increase the ash fusion temperature of the coal enabling the gasifiers to operate at higher temperatures. Fuel 86(17–18):2728–2735
Wall TF, Liu GS (2002) The effects of pressure on coal reactions during pulverised coal combustion and gasification. Prog Energy Combust Sci 28(5):405–433
Wang J, Jiang M (2009) Steam gasification of coal char catalyzed by K2CO3 for enhanced production of hydrogen without formation of methane. Fuel 88(9):1572–1579
Wu Y, Wang J (2011) Potassium-catalyzed steam gasification of petroleum coke for H2 production: reactivity, selectivity and gas release. Fuel Process Technol 92(3):523–530
Ye DP, Agnew JB (1998) Gasification of a South Australian low-rank coal with carbon dioxide and steam: kinetics and reactivity studies. Fuel 77(11):1209–1219
Zhang L-X, Kudo S (2013) Catalytic effects of Na and Ca from inexpensive materials on in-situ steam gasification of char from rapid pyrolysis of low rank coal in a drop-tube reactor. Fuel Process Technol 113:1–7
Zhang J, Zhang L (2015a) Effect of bauxite additives on ash sintering characteristics during the K2CO3-catalyzed steam gasification of lignite. RSC Adv 5(9):6720–6727
Zhang J-G, Zhang L (2015b) Ash Sintering behavior of lignite in catalyzed steam gasification with kaolin as additive. Energy Technol 3(6):556–562
Zhao Y, Zhang W (2018) Kinetic characteristics of in-situ char-steam gasification following pyrolysis of a demineralized coal. Int J Hydrogen Energy 43(24):10991–11001
Zhao Y, Feng D, Zhang Y, Huang Y, Sun S (2016) Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Process Technol 141(Part 1):54–60
Acknowledgements
This work was supported by the Foundation of State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering (Grant No. 2017-K01), and National Natural Science Foundation of China (Grant No. 51706028).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Tsinghua University Press.
About this paper
Cite this paper
Li, J., Zhang, Z., Hao, J., Zhang, J., Zhu, M., Zhang, D. (2022). Effect of K2CO3 Addition on CO2 Gasification Characteristics and Ash Sintering Behaviour of a Chinese Lignite at Different Temperatures and Pressures as Examined Using a High-Pressure Thermogravimetric Analyser. In: Lyu, J., Li, S. (eds) Clean Coal and Sustainable Energy. ISCC 2019. Environmental Science and Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-1657-0_13
Download citation
DOI: https://doi.org/10.1007/978-981-16-1657-0_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1656-3
Online ISBN: 978-981-16-1657-0
eBook Packages: EnergyEnergy (R0)