Abstract
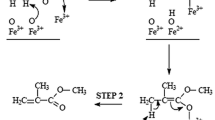
Aerobic oxidative esterification of methacrolein in methanol to methyl methacrylate (MMA) proceeds with high efficiency in the presence of molecular oxygen, on supported gold–nickel oxide (Au–NiOx) nanoparticle catalysts. The method is environmentally benign because it requires only molecular oxygen as the terminal oxidant and gives water as the side product. The Au–NiOx nanoparticles have a core–shell structure, with the Au nanoparticles at the core and the surface covered by highly oxidized NiOx. The Au–NiOx nanoparticles are supported on a carrier with high dispersion. This novel bimetallic nanoparticle structure provides superior catalytic performance than monometallic nanoparticles. Furthermore, we established the industrial catalytic technology with long catalyst life by developing a high-strength silica-based carrier, using NiOx for improved chemical stability, and precisely controlling the distribution of the nanoparticles in the catalysts. The practical applicability of this catalytic system was verified in a 100,000 ton/year MMA production plant in 2008. This process confirmed the high selectivity, high activity, and long life of the Au–NiOx catalyst. This catalyst would help in saving energy and resources, in addition to being highly economical.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Otera J (2003) Esterification: methods, reaction and applications. Wiley-VCH, Weinheim, Germany
Dehydrogenaton (a) Murahashi S-I, Naota T, Ito K, Maeda Y, Taki H (1987) J Org Chem 52:4319–4327. Oxidation with hydrogen peroxide (b) Gopinath R, Patel BK (2000) Org Lett 2:577–579. (c) Wu X-F, Darcel C (2009) Eur J Org Chem 1144–1147. (d) Gopinath R, Barkakaty B, Talukdar B, Patel BK (2003) J Org Chem 68:2944–2947. Oxidation with TBHP (e) Hashmi ASK, Lothschuetz C, Ackermann M, Doepp R, Anantharaman S, Marchetti B, Bertagnolli H, Rominger F (2010) Chem Eur J 16:8012–8019. Oxidation with benzyl chloride (f) Liu C, Tang S, Zheng L, Liu D, Zhang H, Lei A (2012) Angew Chem Int Ed 51:5662–5666. Reviews (g) Ekoue-Kovi K, Wolf C (2008) Chem Eur J 14:6302–6315
(a) Haruta M, Kobayashi T, Sano H, Yamada N (1987) Chem Lett 405–408. (b) Haruta M, Yamada N, Kobayashi T, Iijima S (1989) J Catal 115:301–309
Reviews (a) Hashmi ASK, Hutchings GJ (2006) Angew Chem Int Ed 45:7896–7936. (b) Arcadi A (2008) Chem Rev 108:3266–3325. (c) Li Z, Brouwer C, He C (2008) Chem Rev 108:3239–3265. (d) Pina CD, Falletta E, Prati L, Rossi M (2008) Chem Soc Rev 37:2077–2095. (e) Corma A, Garcia H (2008) Chem Soc Rev 37:2096–2126
(a) Marsden C, Taarning E, Hansen D, Johansen L, Klitgaard SK, Egeblad K, Christensen CH (2008) Green Chem 10:168–170. (b) Fristrup P, Johansen LB, Christensen CH (2008) Chem Commun 2750–2752. (c) Su F-Z, Ni J, Sun H, Cao Y, He H-Y, Fan K-N (2008) Chem Eur J 14:7131–7135. (d) Xu B, Liu X, Haubrich J, Friend CM (2009) Nat Chem 2:61–65
(a) Hayashi T, Inagaki T, Itayama N, Baba H (2006) Catal Today 117:210–213. (b) Nielsen IS, Taarning E, Egeblad K, Madsen R, Christensen CH (2007) Catal Lett 116:35–40. (c) Oliveira RL, Kiyohara PK, Rossi LM (2009) Green Chem 11:1366–1370. (d) Casanova O, Iborra S, Corma A (2009) J Catal 265:109–116. (e) Miyamura H, Yasukawa T, Kobayashi S (2010) Green Chem 12:776–778. (f) Costa VV, Estrada M, Demidova Y, Prosvirin I, Kriventsov V, Cotta RF, Fuentes S, Simakov A, Gusevskaya EV (2012) J Catal 292:148–156. (g) Kotionova T, Lee C, Miedziak P, Dummer NF, Willock DJ, Carley AF, Morgan DJ, Knight DW, Taylor SH, Hutchings G (2012) J Catal Lett 142:1114–1120
Suzuki K, Yamaguchi T, Matsushita K, Iitsuka C, Miura J, Akaogi T, Ishida H (2013) ACS Catal 3:1845–1849
Nagai K (2001) Appl Catal A 221:367–377
(a) Yamamatsu S, Yamaguchi T, Yokota K, Nagano O, Chono M, Aoshima A (2010) Catal Surv Asia 14:124–131. (b) Diao Y, Yan R, Zhang S, Yang P, Li Z, Wang L, Dong H (2009) J Mol Catal A Chem 303:35–42. (c) Wang B, Sun W, Zhu J, Ran W, Chen S (2012) Ind Eng Chem Res 51:15004–15010
Nakagawa K, Konaka R, Nakata T (1962) J Org Chem 27:1597–1601
(a) Choudary BM, Kantam ML, Rahman A, Reddy ChV, Rao KK (2005) Angew Chem Int Ed 40:763–766. (b) Ji H, Wang T, Zhang M, She Y, Wang L (2005) Appl Catal A 282:25–30
Ferreira FF, Fantini MCA (2003) J Phys D Appl Phys 36:2386–2392
The reaction of 1a (15 mmol) in the presence of NiO2·nH2O (0.3 g) in methanol at 80 °C under an oxygen-nitrogen mixture (7:93 (v/v), 3 MPa) for 2 h gave a trace of 2a (1.5 μmol)
Mihaylov M, Knoezinger H, Hadjiivanov K, Gates BC (2007) Chem Ing Tech 79:795–806
Estrella Platero E, Scarano D, Zecchina A, Meneghini G, De Franceschi R (1996) Surf Sci 350:113–122
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Suzuki, K. (2021). Aerobic Oxidative Esterification of Aldehydes with Alcohols by Gold–Nickel Oxide Nanoparticle Catalysts with a Core–Shell Structure. In: Yamashita, H., Li, H. (eds) Core-Shell and Yolk-Shell Nanocatalysts. Nanostructure Science and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-16-0463-8_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-0463-8_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0462-1
Online ISBN: 978-981-16-0463-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)