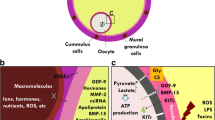
Abstract
As the mammalian oviduct is the place where the fertilization and preimplantation embryo development occur, the oviduct epithelial cells create a favorable environment for gamete/embryo development by secreting various growth factors. Recent research has focused on the important role of oviduct and its derivative for improving the fertilization as well as preimplantation embryo development. In particular, oviduct-derived exosomes have emerged as potential biomarkers for mediating cell-to-cell communication and transporting various genetic materials to recipient cells. According to many recent studies in reproduction field, the role of oviduct-derived exosomes on sperm and early embryo development is well established. However, there is still a lack of information on the effect and function of oviduct-derived exosomes on cumulus-oocyte complexes (COCs). Especially, the oviduct plays an important role for oocyte maturation process in canine species compared with other mammals, because when immature oocytes are ovulated from follicles, canine oocytes undergo maturation processes within the oviduct, while the other mammals already possess matured oocytes in preovulatory follicles. Also, none of the studies have investigated the relationship between oviduct-derived exosomes with COCs, except canine species. Therefore, this chapter focuses on the interaction between oviduct-derived exosomes and cumulus-oocyte complexes in dogs.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AREG:
-
Amphiregulin
- COCs:
-
Cumulus-oocyte complexes
- DNA:
-
Deoxyribonucleic acid
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EREG:
-
Epiregulin
- EVs:
-
Extracellular vesicles
- FBS:
-
Fetal bovine serum
- IVM:
-
In vitro maturation
- MAPK:
-
Mitogen-activated protein kinase
- miRNA:
-
Micro messenger ribonucleic acid
- mRNA:
-
Messenger ribonucleic acid
- NTA:
-
Nanoparticle tracking analysis
- PBS:
-
Phosphate buffer saline
- TEM:
-
Transmission electron microscopy
References
Al-Dossary AA, Bathala P, Caplan JL, Martin-DeLeon PA (2015) Oviductosome-sperm membrane interaction in cargo delivery: detection of fusion and underlying molecular players using three-dimensional super-resolution structured illumination microscopy (SR-SIM). J Biol Chem 290:17710–17723
Al-Dossary AA, Martin-Deleon PA (2016) Role of exosomes in the reproductive tract oviductosomes mediate interactions of oviductal secretion with gametes/early embryo. Front Biosci (Landmark Ed) 21:1278–1285
Al-Dossary AA, Strehler EE, Martin-Deleon PA (2013) Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One 8:e80181
Alcantara-Neto AS, Fernandez-Rufete M, Corbin E et al (2019) Oviduct fluid extracellular vesicles regulate polyspermy during porcine invitro fertilisation. Reprod Fertil Dev
Alminana C, Corbin E, Tsikis G et al (2017) Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction 154:153–168
Alminana C, Heath PR, Wilkinson S et al (2012) Early developing pig embryos mediate their own environment in the maternal tract. PLoS One 7:e33625
Aviles M, Gutierrez-Adan A, Coy P (2010) Oviductal secretions: will they be key factors for the future ARTs? Mol Hum Reprod 16:896–906
Bang C, Thum T (2012) Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 44:2060–2064
Bathala P, Fereshteh Z, Li K, Al-Dossary AA, Galileo DS, Martin-DeLeon PA (2018) Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: murine OVS play a pivotal role in sperm capacitation and fertility. Mol Hum Reprod 24:143–157
Budna-Tukan J, Swiatly-Blaszkiewicz A, Celichowski P et al (2019) “Biological Adhesion” is a significantly regulated molecular process during long-term primary in vitro culture of oviductal epithelial cells (Oecs): a transcriptomic and proteomic study. Int J Mol Sci 20:3387
Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE (2014) Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One 9:e90913
Burns GW, Brooks KE, Spencer TE (2016) Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod 94:56
Carletti MZ, Christenson LK (2009) Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction 137:843–855
Conti M (2010) Signaling networks in somatic cells and oocytes activated during ovulation. Ann Endocrinol (Paris) 71:189–190
Coy P, Garcia-Vazquez FA, Visconti PE, Aviles M (2012) Roles of the oviduct in mammalian fertilization. Reproduction 144:649–660
Cuman C, Menkhorst E, Winship A et al (2014) Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction 147:R75–R86
da Silveira JC, Carnevale EM, Winger QA, Bouma GJ (2014) Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod Biol Endocrinol 12:44
da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ (2012) Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod 86:71
Di Pietro C (2016) Exosome-mediated communication in the ovarian follicle. J Assist Reprod Genet 33:303–311
Diez-Fraile A, Lammens T, Tilleman K et al (2014) Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil (Camb) 17:90–98
Eppig JJ (1991) Intercommunication between mammalian oocytes and companion somatic cells. BioEssays 13:569–574
Fan HY, O’Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS (2010) Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol 24:1529–1542
Fereshteh Z, Schmidt SA, Al-Dossary AA et al (2018) Murine oviductosomes (OVS) microRNA profiling during the estrous cycle: delivery of OVS-borne microRNAs to sperm where miR-34c-5p localizes at the centrosome. Sci Rep 8:16094
Ferraz M, Carothers A, Dahal R, Noonan MJ, Songsasen N (2019) Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci Rep 9:9484
Georgiou AS, Snijders AP, Sostaric E et al (2007) Modulation of the oviductal environment by gametes. J Proteome Res 6:4656–4666
Gilchrist RB (2011) Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev 23:23–31
Gould SJ, Raposo G (2013) As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2
Higginbotham JN, Demory Beckler M, Gephart JD et al (2011) Amphiregulin exosomes increase cancer cell invasion. Curr Biol 21:779–786
Holt WV, Fazeli A (2010) The oviduct as a complex mediator of mammalian sperm function and selection. Mol Reprod Dev 77:934–943
Hsieh M, Thao K, Conti M (2011) Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One 6:e21574
Huang A, Isobe N, Yoshimura Y (2017) Changes in localization and density of CD63-positive exosome-like substances in the hen oviduct with artificial insemination and their effect on sperm viability. Theriogenology 101:135–143
Hung WT, Hong X, Christenson LK, McGinnis LK (2015) Extracellular vesicles from bovine follicular fluid support cumulus expansion. Biol Reprod 93:117
Hunter RH (2005) Fallopian tube physiology: preliminaries to monospermic fertilization and cellular events post-fertilization. Ernst Schering Res Found Workshop:245–261
Hwang SU, Kim KJ, Kim E et al (2018) Lysophosphatidic acid increases in vitro maturation efficiency via uPA-uPAR signaling pathway in cumulus cells. Theriogenology 113:197–207
Jodar M (2019) Sperm and seminal plasma RNAs: what roles do they play beyond fertilization? Reproduction 158:R113–R123
Kalluri R, LeBleu VS (2016) Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harb Symp Quant Biol 81:275–280
Kervancioglu ME, Saridogan E, Atasu T et al (1997) Human Fallopian tube epithelial cell co-culture increases fertilization rates in male factor infertility but not in tubal or unexplained infertility. Hum Reprod 12:1253–1258
Kidson A, Schoevers E, Langendijk P, Verheijden J, Colenbrander B, Bevers M (2003) The effect of oviductal epithelial cell co-culture during in vitro maturation on sow oocyte morphology, fertilization and embryo development. Theriogenology 59:1889–1903
Lange-Consiglio A, Perrini C, Albini G et al (2017) Oviductal microvesicles and their effect on in vitro maturation of canine oocytes. Reproduction 154:167–180
Lee SH, Oh HJ, Kim MJ et al (2017) Oocyte maturation-related gene expression in the canine oviduct, cumulus cells, and oocytes and effect of co-culture with oviduct cells on in vitro maturation of oocytes. J Assist Reprod Genet 34:929–938
Lee SH, Oh HJ, Kim MJ, Setyawan EMN, Lee BC (2019) Interaction of the EGFR signaling pathway with porcine cumulus oocyte complexes and oviduct cells in a coculture system. J Cell Physiol 234:4030–4043
Lee SH, Oh HJ, Kim MJ, Lee BC (2020a) Exosomes derived from oviduct cells mediate the EGFR/MAPK signaling pathway in cumulus cells. J Cell Physiol 235:1386–1404
Lee SH, Oh HJ, Kim MJ, Lee BC (2020b) Canine oviductal exosomes improve oocyte development via EGFR/MAPK signaling pathway. Reproduction 160:613–625
Leese HJ, Hugentobler SA, Gray SM et al (2008) Female reproductive tract fluids: composition, mechanism of formation and potential role in the developmental origins of health and disease. Reprod Fertil Dev 20:1–8
Lopera-Vasquez R, Hamdi M, Fernandez-Fuertes B et al (2016) Extracellular vesicles from BOEC in in vitro embryo development and quality. PLoS One 11:e0148083
Lopera-Vasquez R, Hamdi M, Maillo V et al (2017) Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 153:461–470
Luddi A, Zarovni N, Maltinti E et al (2019) Clues to non-invasive implantation window monitoring: isolation and characterisation of endometrial exosomes. Cell 8
Luvoni GC, Chigioni S, Allievi E, Macis D (2005) Factors involved in vivo and in vitro maturation of canine oocytes. Theriogenology 63:41–59
Maillo V, Gaora PO, Forde N et al (2015) Oviduct-embryo interactions in cattle: two-way traffic or a one-way street? Biol Reprod 92:144
Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ (2016) MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol 7:231
Martinez RM, Liang L, Racowsky C et al (2018) Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci Rep 8:17036
Matsuno Y, Kanke T, Maruyama N, Fujii W, Naito K, Sugiura K (2019) Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS One 14:e0217760
McGough IJ, Vincent JP (2016) Exosomes in developmental signalling. Development 143:2482–2493
Murdica V, Giacomini E, Alteri A et al (2019) Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil Steril 111(897–908):e892
Nakano S, Yamamoto S, Okada A et al (2017) Role of extracellular vesicles in the interaction between epithelial and mesenchymal cells during oviductal ciliogenesis. Biochem Biophys Res Commun 483:245–251
No J, Zhao M, Lee S, Ock SA, Nam Y, Hur TY (2018) Enhanced in vitro maturation of canine oocytes by oviduct epithelial cell co-culture. Theriogenology 105:66–74
Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M (2004) EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684
Perez-Cerezales S, Ramos-Ibeas P, Acuna OS et al (2018) The oviduct: from sperm selection to the epigenetic landscape of the embryo. Biol Reprod 98:262–276
Prochazka R, Blaha M, Nemcova L (2012) Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig. Reproduction 144:535–546
Qu P, Zhao Y, Wang R et al (2019) Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod Fertil Dev 31:324–332
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Saadeldin IM, Kim SJ, Choi YB, Lee BC (2014) Improvement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication. Cell Reprogram 16:223–234
Sahin U, Weskamp G, Kelly K et al (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164:769–779
Saint-Dizier M, Marnier C, Tahir MZ et al (2014) OVGP1 is expressed in the canine oviduct at the time and place of oocyte maturation and fertilization. Mol Reprod Dev 81:972–982
Sang Q, Yao Z, Wang H et al (2013) Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab 98:3068–3079
Schmaltz-Panneau B, Cordova A, Dhorne-Pollet S et al (2014) Early bovine embryos regulate oviduct epithelial cell gene expression during in vitro co-culture. Anim Reprod Sci 149:103–116
Schneider MR, Wolf E (2009) The epidermal growth factor receptor ligands at a glance. J Cell Physiol 218:460–466
Schorey JS, Bhatnagar S (2008) Exosome function: from tumor immunology to pathogen biology. Traffic 9:871–881
Sekiguchi T, Mizutani T, Yamada K et al (2004) Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol 33:281–291
Sela-Abramovich S, Chorev E, Galiani D, Dekel N (2005) Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146:1236–1244
Singh AB, Harris RC (2005) Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal 17:1183–1193
Sohel MM, Hoelker M, Noferesti SS et al (2013) Exosomal and non-exosomal transport of extra-cellular micrornas in follicular fluid: implications for bovine oocyte developmental competence. PLoS One 8:e78505
Songsasen N, Wildt DE (2007) Oocyte biology and challenges in developing in vitro maturation systems in the domestic dog. Anim Reprod Sci 98:2–22
Stremersch S, De Smedt SC, Raemdonck K (2016) Therapeutic and diagnostic applications of extracellular vesicles. J Control Release 244:167–183
Taverna S, Pucci M, Giallombardo M et al (2017) Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep 7:3170
Vyas P, Balakier H, Librach CL (2019) Ultrastructural identification of CD9 positive extracellular vesicles released from human embryos and transported through the zona pellucida. Syst Biol Reprod Med 65:273–280
Whiteside TL (2016) Exosomes and tumor-mediated immune suppression. J Clin Invest 126:1216–1223
Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13:269–288
Yanez-Mo M, Siljander PR, Andreu Z et al (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066
Zumoffen CM, Massa E, Caille AM, Munuce MJ, Ghersevich SA (2015) Effects of lactoferrin, a protein present in the female reproductive tract, on parameters of human sperm capacitation and gamete interaction. Andrology 3:1068–1075
Acknowledgments
The authors would like to acknowledge the Research Institute for Veterinary Science and the BK21 plus program and global PhD Fellowship Program through NRF who provided insight and expertise that greatly assisted the research.
Funding: This research was supported by the global PH.D Fellowship Program through NRF funded by the Ministry of Education (NRF-20142A1021187).
Disclosure of interests: All authors declare they have no conflict of interest.
Ethical approval for studies involving humans: This article does not contain any studies with human participants performed by any of the authors.
Ethical approval for studies involving animals: Animal experiments were performed following a standard procedure established by the Committee for Accreditation of Laboratory Animal Care and the Guideline for the Care and Use of Laboratory Animals of Seoul National University (approval number; SNU-140704-1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lee, S.H., Lee, B.C. (2021). The Interplay Between Oviduct-Derived Exosomes and Cumulus-Oocyte Complexes. In: Alzahrani, F.A., Saadeldin, I.M. (eds) Role of Exosomes in Biological Communication Systems. Springer, Singapore. https://doi.org/10.1007/978-981-15-6599-1_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-6599-1_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6598-4
Online ISBN: 978-981-15-6599-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)