Abstract
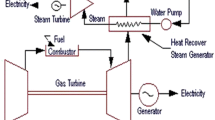
This chapter analyses syngas production through pyrolysis and gasification, its compression and its use in gas turbines. Syngas compression can be performed during or after thermal treatment processes. Important points are discussed related to syngas ignition, syngas explosion limit at high temperatures and high pressures and syngas combustion kinetics. Kinetic aspects influence ignition and final emissions which are obtained at the completion of the combustion process. The chapter is organized into four subsections, dealing with (1) innovative syngas production plants, (2) syngas compressors and compression process, (3) syngas ignition in both heterogeneous and homogeneous systems and (4) syngas combustion kinetics and experimental methods. Particular attention is given to ignition regions that affect the kinetics, namely systems that operate at temperatures higher than 1000 K can have strong ignition, whereas those operating at lower temperatures have weak ignition.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
D’Alessandro B, D'Amico M, Desideri U, Francesco F. The IPRP (integrated pyrolysis regenerated plant) technology: from concept to demonstration. Appl Energy. 2013;101(1):423–31. https://doi.org/10.1016/j.apenergy.2012.04.036.
Zhang X, Che Q, Cui X, Wei Z, Zhang X, Chen Y, Wang X, Chen H. Application of biomass pyrolytic polygeneration by a moving bed: characteristics of products and energy efficiency analysis. Bioresour Technol. 2018;254:130–8. https://doi.org/10.1016/j.biortech.2018.01.083.
Minchener AJ. Coal gasification for advanced power generation. Fuel. 2005;84(17):2222–35. https://doi.org/10.1016/j.fuel.2005.08.035.
Beaudin M, Zareipour H, Schellenberglabe A, Rosehart W. Energy storage for mitigating the variability of renewable electricity sources: an updated review. Energy Sustain Dev. 2010;14(4):302–14. https://doi.org/10.1016/j.esd.2010.09.007.
Tukiainen S. Carbofex EBC-certified biochar technology, Carbofex. 2020. https://www.carbofex.fi/. Accessed 18 Jan 2020.
Carbon Terra. Das unternehmen. 2020. https://www.carbon-terra.eu/en/schottdorf-meiler/functionality. Accessed 18 Jan 2020.
Gustafsson M. 2013Pyrolysis for heat production. Master thesis. 2013. http://www.diva-portal.org/smash/get/diva2:655188/FULLTEXT02.pdf. Accessed 18 Jan 2020.
EliquoStulz. PYREG, Minimise the cost of your sludge disposal. 2020. https://www.eliquostulz.com/en/pyreg.html. Accessed 18 Jan 2020.
Ahmad E, Jäger N, Apfelbacher A, Daschner R, Hornung A, Pant KK. Integrated thermo-catalytic reforming of residual sugarcane bagasse in a laboratory scale reactor. Fuel Process Technol. 2018;171:277–86. https://doi.org/10.1016/j.fuproc.2017.11.020.
3R Agrocarbon. The 3R zero emission pyrolysis technology generates new resources and added value recovered BIO-PHOSPHATE products. 2020. https://www.3ragrocarbon.com/sites/default/files/attachments/3r_fact_sheet.pdf. Accessed 18 Jan 2020
Rietveld G, van der Drift A, Grootjes AJ, van der Meijden CM, Vreugdenhil BJ. Commercialization of the ECN MILENA gasification technology, ECN report ECN-M--14-061. 2014. https://publications.tno.nl/publication/34631595/N4tX73/m14061.pdf. Accessed 18 Jan 2020.
Ahrenfeldt J, Thomsen TP, Henriksen U, Clausen LR. Biomass gasification cogeneration – a review of state of the art technology and near future perspectives. Appl Therm Eng. 2013;50(2):1407–17. https://doi.org/10.1016/j.applthermaleng.2011.12.040.
Hofbauer H, Rauch R, Bosch K, Koch R, Aichernig C. Biomass CHP plant güssing - a success story. 2002. https://www.semanticscholar.org/paper/Biomass-CHP-Plant-G%C3%BCssing-A-Success-Story-Hermann-Reinhard/0122da66ae2088ad3f046a150f251a4a345b8038. Accessed 18 Jan 2020.
Biollaz S. The SNG technology platform in güssing, a status report of bio-SNG project. In: Poster presented at European Biofuels Technology Platform: Second Stakeholder Plenary Meeting (SPM2), 22nd of January 2009, Brussels. 2009. http://www.etipbioenergy.eu/images/Poster_BioSNG_PSI.pdf. Accessed 18 Jan 2020.
Alamia A, Magnusson I, Johnsson F, Thunman H. Well-to-wheel analysis of bio-methane via gasification, in heavy duty engines within the transport sector of the European Union. Appl Energy. 2016;170:445–54. https://doi.org/10.1016/j.apenergy.2016.02.001.
Hedenskog M. The GoBiGas project: bio-methane from forest residues – from vision to reality. Presentation at SVEBIO2015. 2015.
Alamia A, Larsson A, Breitholtz C, Thunman H. Performance of large-scale biomass gasifiers in a biorefinery, a state-of-the-art reference. Int J Energy Res. 2017;41:2001–19. https://doi.org/10.1002/er.3758.
Larsson A, Hedenskog M, Thunman H. Monitoring the bed material activation in the GoBiGas-Gasifier. In: Nordic Flame days. 2015. https://www.researchgate.net/publication/283578378_Monitoring_the_Bed_Material_Activation_in_the_GoBiGas-Gasifier. Accessed 18 Jan 2020.
Rauch R, Hofbauer H, Bosch K, Siefert I, Aichernig C, Voigtlaender K et al. Steam gasification of biomass at CHP plant guessing-status of the demonstration plant. In: World biomass conference; biomass for energy industry and climate protection, pp 1687–1690. 2004.
Pfeifer C, Koppatz S, Hofbauer H. Steam gasification of various feedstocks at a dual fluidised bed gasifier: impacts of operation conditions and bed materials. Biomass Convers Biorefin. 2011;1:39–53. https://doi.org/10.1007/s13399-011-0007-1.
Kotik J. Über den Einsatz von Kraft-Wärme-Kopplungsanlagen auf basis der wirbelschichtdampfvergasung fester biomasse am beispiel des biomassekraftwerks oberwart. Vienna: Vienna University of Technology; 2010.. (in German)
Suda T, Liu Z, Takafuji M, Hamada K, Tani H. Gasification of lignite coal and biomass using twin IHI Gasifier (TIGAR®). 2012. https://www.ihi.co.jp/var/ezwebin_site/storage/original/application/fae45aac0eb82bef2ca20cf8cc2cb0f0.pdf. Accessed 18 Jan 2020.
Paethanom A. Twin IHI Gasifier (TIGAR®) – current status of Indonesian demonstration project and its business plan. In gasification and syngas technologies conference. Vancouver, BC. 2016. https://www.globalsyngas.org/members/2016-conference-presentations/. Accessed 18 Jan 2020.
Melligan F, Auccaise R, Novotny EH, Leahy JJ, Hayes MHB, Kwapinski W. Pressurised pyrolysis of Miscanthus using a fixed bed reactor. Bioresour Technol. 2011;102(3):3466–70. https://doi.org/10.1016/j.biortech.2010.10.129.
Mahinpey N, Murugan P, Mani T, Raina R. Analysis of bio-oil, biogas, and biochar from pressurized pyrolysis of wheat straw using a tubular reactor. Energy Fuel. 2009;23:2736–42. https://doi.org/10.1021/ef8010959.
Fjellerup J, Gjernes E, Hansen LK. Pyrolysis and combustion of pulverized wheat straw in a pressurized entrained flow reactor. Energy Fuel. 1996;10:649–51. https://doi.org/10.1021/ef950204e.
Whitty K, Backman R, Hupa M. Influence of pressure on pyrolysis of black liquor: 1. Swelling. Bioresour Technol. 2008;99:663–70. https://doi.org/10.1016/j.biortech.2006.11.065.
Roberts DG, Harris DJ, Wall TF. On the effects of high pressure and heating rate during coal pyrolysis on char gasification reactivity. Energy Fuel. 2003;17:887–95. https://doi.org/10.1021/ef020199w.
Matsuoka K, Akiho H, Xu WC, Gupta R, Wall TF, Tomita A. The physical character of coal char formed during rapid pyrolysis at high pressure. Fuel. 2005;84:63–9. https://doi.org/10.1016/j.fuel.2004.07.006.
Vuthaluru HB. Investigations into the pyrolytic behaviour of coal/biomass blends using thermogravimetric analysis. Bioresour Technol. 2004;92:187–95. https://doi.org/10.1016/j.biortech.2003.08.008.
Cetin E, Gupta R, Moghtaderi B. Effect of pyrolysis pressure and heating rate on radiata pine char structure and apparent gasification reactivity. Fuel. 2005;84:1328–34. https://doi.org/10.1016/j.fuel.2004.07.016.
Porada S. The influence of elevated pressure on the kinetics of evolution of selected gaseous products during coal pyrolysis. Fuel. 2004;83(7–8):1071–8. https://doi.org/10.1016/j.fuel.2003.11.004.
Blackmer®. Compressors, 04/99 CB-207. 2020. https://www.gasequipment.com/catalogs/cryogenic/pdf/Blackmer/Compressors/Comp%20Selection%20and%20Sizing.pdf. Accessed on 17 Jan 2020.
Process Industry Practices Machinery. Compressor selection guidelines. 2013. https://pip.org/docs/default-source/practices-documents/reec001bf1ca80395a262f789edff00008ddc6a.pdf?sfvrsn=d3beca9e_0. Accessed 17 Jan 2020.
Gresh MT. Compressor performance: aerodynamics for the user. Amsterdam: Elsevier; 2001.
Rezvani S, McIlveen-Wright D, Huang Y, Dave A, Deb Mondol J, Hewitt N. Comparative analysis of energy storage options in connection with coal fired integrated gasification combined cycles for an optimized part load operation. Energy Convers Manag. 2012;101:154–60. https://doi.org/10.1016/j.fuel.2011.07.034.
Amos WA. Costs of storing and transporting hydrogen. National Renewable Energy Lab, Golden, CO. 1999. https://www.nrel.gov/docs/fy99osti/25106.pdf. Accessed 17 Jan 2020.
Cau G, Cocco D, Serra F. Energy and cost analysis of small-size integrated coal gasification and syngas storage power plants. Energy Convers Manag. 2012;56:121–9. https://doi.org/10.1016/j.enconman.2011.11.025.
Richards GA, McMillian MM, Gemmen RS, Rogers WA, Cully SR. Issues for low-emission, fuel-flexible power systems. Prog Energy Combust Sci. 2001;27(2):141–69. https://doi.org/10.1016/S0360-1285(00)00019-8.
Lieuwen T, McDonell V, Santavicca D, Sattelmayer T. Burner development and operability issues associated with steady flowing syngas fired combustors. Combust Sci Technol. 2008;180(6):1169–92. https://doi.org/10.1080/00102200801963375.
He F, Li Z, Liu P, Ma L, Pistikopoulos EN. Operation window and part-load performance study of a syngas fired gas turbine. Appl Energy. 2012;89(1):133–41. https://doi.org/10.1016/j.apenergy.2010.11.044.
Frey HC, Zhu Y. Improved system integration for integrated gasification combined cycle (IGCC) systems. Environ Sci Technol. 2006;40:1693–9. https://doi.org/10.1021/es0515598.
Smith AR, Klosek J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process Technol. 2001;70(2):115–34. https://doi.org/10.1016/S0378-3820(01)00131-X.
Geosits RF, Schmoe Lee A. IGCC – the challenges of integration. In: Proceedings of GT2005 ASME turbo expo 2005: power for land, sea, and air, Reno, NV. 2005. https://www.edockets.state.mn.us/EFiling/edockets/searchDocuments.do?method=showPoup&documentId={1AB68706-28A0-4706-B9A8-5C412EAF4BDE}&documentTitle=281454. Accessed 17 Jan 2020.
Jaeger H. Plant design net rated 644 MW and 38% HHV on low rank coal, gas turbine world; March–April 2006. 2006.
Lee C, Lee SJ, Yun Y. Effect of air separation unit integration on integrated gasification combined cycle performance and NOx emission characteristics. Korean J Chem Eng. 2007;24(2):368–73. https://doi.org/10.1007/s11814-007-5047-7.
NATO. Performance prediction and simulation of gas turbine engine operation for aircraft, marine, vehicular, and power generation. Technical report RTO-TRAVT-036. Research and Technology Organisation, North Atlantic Treaty Organisation. 2007. https://apps.dtic.mil/docs/citations/ADA466188. Accessed 17 Jan 2020.
Gupta KK, Rehman A, Sarviya RM. Bio-fuels for the gas turbine: a review. Renew Sustain Energy Rev. 2010;14(9):2946–55. https://doi.org/10.1016/j.rser.2010.07.025. Accessed 17 Jan 2020
Todd DM, Battista RA. Demonstrated applicability of hydrogen fuel for gas turbines. In: Proceedings of gasification for the future, Noordwijk, Nederland. 2000.
Shilling N, Jones RM. The response of gas turbines to a CO2 constrained environment. In: Gasification technology conference report, GE Power Systems.
Chiesa P, Lozza G, Mazzocchi L. Using hydrogen as gas turbine fuel. Trans ASME J Eng Gas Turb Power. 2005;127(1):73–80. https://doi.org/10.1115/1.1787513.
Gardiner WC Jr, McFarland M, Morinaga K, Takeyama T, Walker BF. Initiation rate for shock-heated hydrogen-oxygen-carbon monoxide-argon mixtures as determined by OH induction time measurements. J Phys Chem. 1971;75:1504–9. https://doi.org/10.1021/j100680a022.
Dean AM, Steiner DL, Wang EE. A shock tube study of the H2/O2/CO/Ar and H2/N2O/CO/Ar systems: measurement of the rate constant for H + N2O = N2 + OH combust. Flame. 1978;32:73–83. https://doi.org/10.1016/0010-2180(78)90081-0.
Fotache CG, Tan Y, Sung CJ, Law CK. Ignition of CO/H2/N2 versus heated air in counterflow: experimental and modeling results. Combust Flame. 2000;120:417–26. https://doi.org/10.1016/S0010-2180(99)00098-X.
Wierzba I, Kilchyk V. Flammability limits of hydrogen–carbon monoxide mixtures at moderately elevated temperatures. Int J Hydrogen Energy. 2001;26:639–43. https://doi.org/10.1016/S0360-3199(00)00114-2.
Mueller MA, Yetter RA, Dryer FL. Flow reactor studies and kinetic modeling of the H2/O2/NOX and CO/H2O/O2/NOX reactions. Int J Chem Kinet. 1999;31:705–24. https://doi.org/10.1002/(SICI)1097-4601(1999)31:10<705::AID-JCK4>3.0.CO;2-%23.
Davis SG, Joshi AV, Wang H, Egolfopoulos F. An optimized kinetic model of H2/CO combustion. Proc Combust Inst. 2005;30:1283–92. https://doi.org/10.1016/j.proci.2004.08.252.
Zsely IG, Zador J, Turanyi T. Uncertainty analysis of updated hydrogenand carbon monoxide oxidation mechanisms. Proc Combust Inst. 2005;30:1273–81. https://doi.org/10.1016/j.proci.2004.08.172.
Walton SM, He X, Zigler BT, Wooldridge MS. An experimental investigation of the ignition properties of hydrogen and carbon monoxide mixtures for syngas turbine applications. Proc Combust Inst. 2007;31(2):3147–54. https://doi.org/10.1016/j.proci.2006.08.059.
Donovan MT, He X, Zigler BT, Palmer TR, Wooldridge MS, Atreya A. Demonstration of a free-piston rapid compression facility for the study of high temperature combustion phenomena. Combust Flame. 2004;137(3):351–65. https://doi.org/10.1016/j.combustflame.2004.02.006.
He X, Donovan MT, Zigler BT, Palmer TR, Walton SM, Wooldridge MS, Atreya A. Combust. Flame. 2005;142:266–75. https://doi.org/10.1016/j.combustflame.2005.02.014.
Lee D, Hochgreb S. Hydrogen autoignition at pressures above the second explosion limit (0.6–4.0 MPa). Int J Chem Kinet. 1998;30:385–406. https://doi.org/10.1002/(SICI)1097-4601(1998)30:6<385::AID-KIN1>3.0.CO;2-O.
Elsworth JE, Haskell WW, Read IA. Non-uniform ignition processes in rapid-compression machines combust. Flame. 1969;13(4):437–8. https://doi.org/10.1016/0010-2180(69)90115-1.
Vermeer DJ, Meyer JW, Oppenheim AK. Auto-ignition of hydrocarbons behind reflected shock waves combust. Flame. 1972;18(3):327–36. https://doi.org/10.1016/S0010-2180(72)80183-4.
Fieweger K, Blumenthal R, Adomeit G. Self-ignition of S.I. engine model fuels: a shock tube investigation at high pressure combust. Flame. 1997;109(4):599–619. https://doi.org/10.1016/S0010-2180(97)00049-7.
Walton SM, He X, Zigler BT, Wooldridge MS, Atreya A. Demonstration of distinct ignition regimes using high-speed digital imaging of Iso-octane mixtures. In: Proc. fourth joint meeting of the US Sections of the Combust. Inst. https://ci.confex.com/ci/2005/techprogram/P1498.HTM
Walton SM, He X, Zigler BT, Wooldridge MS, Atreya A. An experimental investigation of iso-octane ignition phenomena. Combust Flame. 2007;150(3):246–62. https://doi.org/10.1016/j.combustflame.2006.07.016.
Wang H. Private communication. 2005.
Chaos M, Dryer FL. Syngas combustion kinetics and applications. Combust Sci Technol. 2008;180(6):1053–96. https://doi.org/10.1080/00102200801963011.
Grogan KP, Ihme M. Weak and strong ignition of hydrogen/oxygen mixtures in shock-tube systems. Proc Combust Inst. 2015;35(2):2181–9. https://doi.org/10.1016/j.proci.2014.07.074.
Voevodsky VV, Soloukhin RI. On the mechanism and explosion limits of hydrogen-oxygen chain self-ignition in shock waves. Proc Combust Inst. 1965;10(1):279–83. https://doi.org/10.1016/S0082-0784(65)80173-4.
Meyer J, Oppenheim A. On the shock-induced ignition of explosive gases. Symp Combust. 1971;13(1):1153–64. https://doi.org/10.1016/S0082-0784(71)80112-1.
Mansfield AB, Wooldridge MS. High-pressure low-temperature ignition behavior of syngas mixtures. Combust Flame. 2014;161(9):2242–51. https://doi.org/10.1016/j.combustflame.2014.03.001.
Frassoldati A, Faravelli T, Ranzi E. The ignition, combustion and flame structure of carbon monoxide/hydrogen mixtures. Note 1: detailed kinetic modeling of syngas combustion also in presence of nitrogen compounds. Int J Hydrog Energy. 2007;32(15):3471–85. https://doi.org/10.1016/j.ijhydene.2007.01.011.
Mittal G, Sung CJ, Yetter RA. Autoignition of H2/CO at elevated pressures in a rapid compression machine. Int J Chem Kinet. 2006;38:516. https://doi.org/10.1002/kin.20180.
Frassoldati A, Faravelli T, Ranzi E. A wide range modeling study of NOx formation and nitrogen chemistry in hydrogen combustion. Int J Hydrogen Energy. 2006;31(15):2310–28. https://doi.org/10.1016/j.ijhydene.2006.02.014.
Kalitan DM, Petersen EL. Ignition and oxidation of lean CO/H2 fuel blends in air 41st AIAA/ASME/SAE/ASEE joint propulsion conference and exhibit, 10–13 July 2005, Tucson, Arizona (USA), paper 2005-3767. 2005. https://doi.org/10.2514/1.28123.
Saxena P, Williams FA. Testing a small detailed chemical-kinetic mechanism for the combustion of hydrogen and carbon monoxide. Combust Flame. 2006;145:316–23. https://doi.org/10.1016/j.combustflame.2005.10.004.
Olm C, Zsély IG, Varga T, Curran HJ, Turányi T. Comparison of the performance of several recent syngas combustion mechanisms. Combust Flame. 2015;162:1793–812. https://doi.org/10.1016/j.combustflame.2014.12.001.
Healy D, Kalitan DM, Aul CJ, Petersen EL, Bourque G, Curran HJ. Oxidation of C1−C5 alkane quinternary natural gas mixtures at high pressures. Energy Fuel. 2010;24:1521–8. https://doi.org/10.1021/ef9011005.
Kéromnès A, Metcalfe WK, Heufer KA, Donohoe N, Das AK, Sung CJ, Herzler J, Naumann C, Griebel P, Mathieu O, Krejci MC, Petersen EL, Pitz WJ, Curran HJ. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust Flame. 2013;160:995–1011. https://doi.org/10.1016/j.combustflame.2013.01.001.
Li J, Zhao Z, Kazakov A, Chaos M, Dryer FL, Scire JJJ. A comprehensive kinetic mechanism for CO, CH2 O, and CH 3 OH combustion. Int J Chem Kinet. 2007;39(3):109–36. https://doi.org/10.1002/kin.20218.
Wang H, You X, Joshi AV, Davis SG, Laskin A, Egolfopoulos F, Law CK. USC Mech version II. High-temperature combustion reaction model of H2/CO/C1-C4 compounds. http://ignis.usc.edu/USC_Mech_II.htm/. Accessed 17 Jan 2020.
Mechanical and Aerospace Engineering (Combustion Research), University of California at San Diego: Chemical-Kinetic Mechanisms for Combustion Applications, San Diego Mechanism, version 2014-02-17. http://combustion.ucsd.edu. Accessed 17 Jan 2020.
CRECK modeling Group Hydrogen/CO mechanism version 1212. http://creckmodeling.chem.polimi.it/kinetic.html/. Accessed 17 Jan 2020.
Li X, You X, Wu F, Law CK. Uncertainty analysis of the kinetic model prediction for high-pressure H2/CO combustion. Proc Combust Inst. 2015;35(1):617–24. https://doi.org/10.1016/j.proci.2014.07.047.
Starik AM, Titova NS, Sharipov AS, Kozlov VE. Syngas oxidation mechanism. Combust. Explos. Shock Waves. 2010;46:491–506. https://doi.org/10.1007/s10573-010-0065-x.
Smith GP, Golden DM, Frenklach M, Moriary NW, Eiteneer B, Goldenberg M, Bowman CT, Hanson RK, Song S, Gardiner WC, Lissianski VV, Qin Z. GRI-Mech 3.0. http://combustion.berkeley.edu/gri-mech/version30/text30.html. Accessed 15 Nov 2019.
Rasmussen CL, Hansen J, Marshall P, Glarborg P. Experimental measurements and kinetic modeling of CO/H2/O2/NOx conversion at high pressure. Int J Chem Kinet. 2008;40:454–80. https://doi.org/10.1002/kin.20327.
Sun H, Yang SI, Jomaas G, Law CK. High-pressure laminar flame speeds and kinetic modeling of carbon monoxide/hydrogen combustion. Proc Combust Inst. 2007;31(1):439–46. https://doi.org/10.1016/j.proci.2006.07.193.
Ahmed SS, Mauß F, Moréac G, Zeuch T. A comprehensive and compact n-heptane oxidation model derived using chemical lumping. Phys Chem Chem Phys. 2007;9:1107–26. https://doi.org/10.1039/B614712G.
Dagaut P, Lecomte F, Mieritz J, Glarborg P. Experimental and kinetic modeling study of the effect of NO and SO2 on the oxidation of CO-H2 mixtures. Int J Chem Kinet. 2003;35(11):564–75. https://doi.org/10.1002/kin.10154.
Wu KT, Lee HT, Juch CI, Wan HP, Shim HS, Adams BR, Chen SL. Study of syngas co-firing and reburning in a coal fired boiler. Fuel. 2004;83(14–15):1991–2000. https://doi.org/10.1016/j.fuel.2004.03.015.
Ranzi E, Sogaro A, Gaffuri P, Pennati G, Faravelli T. A wide range modeling study of methane oxidation. Combust Sci Technol. 1994;96(4–6):279–325. https://doi.org/10.1080/00102209408935359.
Nist best fit. 2006. http://kinetics.nist.gov/index.php. Accessed 15 Nov 2019.
Tsang W, Hampson RF. Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds. J Phys Chem Ref Data. 1986;15(3):1087–279. https://doi.org/10.1063/1.555759.
Timonen RS, Ratajczak E, Gutman D. The addition and dissociation reaction atomic hydrogen + carbon monoxide. dblharw. oxomethyl. 2. Experimental studies and comparison with theory. J Phys Chem. 1987;91:5325. https://doi.org/10.1021/j100304a037.
Jachimowski CJ. Chemical kinetic reaction mechanism for the combustion of propane. Combust Flame. 1984;55(2):213–24. https://doi.org/10.1016/0010-2180(84)90029-4.
Gardiner WCJ, editor. Combustion chemistry. New York: Springer; 1984. https://doi.org/10.1007/978-1-4684-0186-8.
Michael JV, Su MC, Sutherland JW, Carroll JJ, Wagner AF. Rate constants for H + O2 + M f HO2 + M in seven Bath gases. J Phys Chem A. 2002;106:5297–313. https://doi.org/10.1021/jp020229w.
Westbrook CK, Dryer F. Chemical kinetic modeling of hydrocarbon combustion. Prog Energy Combust Sci. 1984;10(1):1–57. https://doi.org/10.1016/0360-1285(84)90118-7.
Peeters J, Mahnew G. Reaction mechanisms and rate constants ofelementary steps in methane-oxygen flames. Symp Combust. 1973;1973(14):133–46. https://doi.org/10.1016/S0082-0784(73)80015-3.
Petersen EL, Davidson DF, Hanson RK. Kinetics modeling of shock-induced ignition in low-dilution CH4/O2 mixtures at high pressures and intermediate temperatures. Combust Flame. 1999;117:272–90. https://doi.org/10.1016/S0010-2180(98)00111-4.
Goodings JM, Hayhurst ANJ. Heat release and radical recombination in premixed fuel-lean flames of H2+ O2+ N2. Rate constants for H + OH + M → H2O + M and HO2+ OH → H2O + O2. Chem Soc Faraday Trans 2. 1988;84:745–62. https://doi.org/10.1039/F29888400745.
Hong Z, Vasu SS, Davidson DF, Hanson RK. Experimental study of the rate of OH + HO2 f H2O + O2 at high temperatures using the reverse reaction. J Phys Chem A. 2010;114:5520–5. https://doi.org/10.1021/jp100739t.
Wooldridge MS, Hanson RK, Bowman CT. A shock tube study of CO + OH → CO2 + H and HNCO + OH → products via simultaneous laser absorption measurements of OH and CO2. Int J Chem Kinet. 1996;28:361–72. https://doi.org/10.1002/(SICI)1097-4601(1996)28:5<361::AID-KIN5>3.0.CO;2-T.
Zhao Z, Li J, Kazakov A, Dryer FL. Temperature-dependent feature sensitivity analysis for combustion modeling. Int J Chem Kinet. 2005;37:282. https://doi.org/10.1002/kin.20080.
Joshi AV, Wang H. Master equation modeling of wide range temperature and pressure dependence of CO + OH → products. Int J Chem Kinet. 2006;38:57. https://doi.org/10.1002/kin.20137.
Wooldridge MS, Hanson RK, Bowman CT. A shock tube study of the CO+OH → CO2 +H reaction. Proc Combust Inst. 1994;25:741–8. https://doi.org/10.1016/S0082-0784(06)80706-X.
Golden DM, Smith GP, McEwen AB, Yu CL, Eitneer B, Frenklach M, Vaghjiani GL, Ravishankara AR, Tully FP. OH (OD) + CO: measurements and an optimized RRKM fit. J Phys Chem A. 1998;102(44):8598–606. https://doi.org/10.1021/jp982110m.
Sun HY, Yang SI, Jomaas G, Law CK. High-pressure laminar flame speeds and kinetic modeling of carbon monoxide/hydrogen combustion. Proc Combust Inst. 2006;31(1):439–46. https://doi.org/10.1016/j.proci.2006.07.193.
Lin MC, Bauer SH. Bimolecular reaction of N2O with CO and the recombination of O and CO as studied in a single-pulse shock tube. J Chem Phys. 1969;50:3377. https://doi.org/10.1063/1.1671561.
Warnatz J. Rate coefficients in the C/H/O system. In: Gardiner Jr WC, editor. Combustion chemistry. New York: Springer; 1984. p. 197–360. https://doi.org/10.1007/978-1-4684-0186-8.
Hardy JW, Gardiner WC Jr, Burcat A. Recombination of carbon monoxide and oxygen atoms. Int J Chem Kinet. 1974;10:503–17. https://doi.org/10.1002/kin.550100508.
Wagner HG, Zabel F, Bunsenges B. Neuere Untersuchungen zum thermischen Zerfall von CO2. Teil II. Phys Chem. 1974;72:705. https://doi.org/10.1002/bbpc.19740780717.
Inn ECY. Rate of recombination of oxygen atoms and CO at temperatures below ambient. J Chem Phys. 1974;61:1589. https://doi.org/10.1063/1.1682139.
Simonaitis R, Heicklen J. Kinetics and mechanism of the reaction of O (3P) with carbon monoxide. J Chem Phys. 1972;56:2004. https://doi.org/10.1063/1.1677490.
Toby S, Sheth S, Toby FS. The chemistry of combustion processes. In: Sloane TM, editor. ACS symposium series, vol. 249. Washington: DC pp; 1984. p. 267–76. https://doi.org/10.1021/bk-1983-0249.ch016.
Kondratiev VN. On the rate of CO + O recombination. React Kinet Catal Lett. 1974;1:7–13. https://doi.org/10.1007/BF02075114.
Kondratiev VN. Proc Combust Inst. 1959;7:41.
Allen MT, Yetter RA, Dryer FL. High pressure studies of moist carbon monoxide/nitrous oxide kinetics. Combust Flame. 1997;109:449–70. https://doi.org/10.1016/S0010-2180(96)00181-2.
Troe J. Thermal dissociation and recombination of polyatomic molecules. Proc Combust Inst. 1975;15(1):667–80. https://doi.org/10.1016/S0082-0784(75)80337-7.
Faravelli T, Frassoldati A, Ranzi E. Kinetic modeling of the interactions between NO and hydrocarbons in the oxidation of hydrocarbons at low temperatures. Combust Flame. 132:188–207. https://doi.org/10.1016/S0010-2180(02)00437-6.
Frassoldati A, Faravelli T, Ranzi E. Kinetic modeling of the interactions between NO and hydrocarbons at high temperature. Combust Flame. 2003;135:97–112. https://doi.org/10.1016/S0010-2180(03)00152-4.
Boivin P, Jiménez C, Sánchez AL, Williams FA. A four-step reduced mechanism for syngas combustion. Combust Flame. 2011;158(6):1059–63. https://doi.org/10.1016/j.combustflame.2010.10.023.
Boivin P, Jiménez C, Sánchez AL, Williams FA. An explicit reduced mechanism for H2–air combustion. Proc Combust Inst. 2011;33(1):517–23. https://doi.org/10.1016/j.proci.2010.05.002.
Burke MP, Chaos M, Dryer FL, Ju Y. Negative pressure dependence of mass burning rates of H2/CO/O2/diluent flames at low flame temperatures. Combust Flame. 2010;157(4):618–31. https://doi.org/10.1016/j.combustflame.2009.08.009.
Burke MP, Chen Z, Ju Y, Dryer FL. Effect of cylindrical confinement on the determination of laminar flame speeds using outwardly propagating flames. Combust Flame. 2009;156(4):771–9. https://doi.org/10.1016/j.combustflame.2009.01.013.
Delattin F, Di Lorenzo G, Rizzo S, Bram S, De Ruyck J. Combustion of syngas in a pressurized microturbine-like combustor: experimental results. Appl Energy. 2010;87(4):1441–52. https://doi.org/10.1016/j.apenergy.2009.08.046.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yang, M. et al. (2020). Syngas Production, Storage, Compression and Use in Gas Turbines. In: Fang, Z., Smith Jr, R.L., Xu, L. (eds) Production of Biofuels and Chemicals with Pyrolysis. Biofuels and Biorefineries, vol 10. Springer, Singapore. https://doi.org/10.1007/978-981-15-2732-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-15-2732-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2731-9
Online ISBN: 978-981-15-2732-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)