Abstract
Despite improvements over the past 30 years, 10-year pediatric heart transplantation survival rates remain low and mechanical support is both expensive and relatively unavailable. In contrast to isolated cell therapies, implantable engineered cardiac tissues (ECTs) recover myocardial mass and function, creating the opportunity for cardiac recovery rather than replacement. Our ECT research has progressed from using embryonic avian and rodent cell compositions to human induced pluripotent stem cell (h-iPSC)-derived, multiple cell lineage formulations with the goal of clinical translation. We generate ECTs from h-iPSC-derived cardiomyocytes (CM), endothelial cells (EC), and vascular mural cells (MC) in both linear (15 × 1 mm) and large format (LF, 20 × 20 mm) geometries using rodent-derived and human-compatible reagents. H-iPSC ECTs undergo rapid gel compaction and begin intrinsic beating by day 3. CM fraction at ECT formation is approximately 60%. The ECT maximum capture rates increased during in vitro culture up to 28 days and in response to optogenetic pacing (OP) using AAV-ChIEF from day 7 to 14. ECT relaxation times decreased, force-frequency relations became more neutral, and beat-to-beat hysteresis decreased with prolonged culture or OP. H-iPSC-derived ECTs generated using human collagen I and human compatible MaxGel have comparable structural and functional features to rodent-derived ECTs. Linear and LF-ECTs implanted onto xenotolerant infarcted rat hearts survived, engrafted, improved ejection fraction, normalized regional echo strain, and reduced scar area at 4 weeks. Thus, these h-iPSC ECT compositions show promise as a strategy for pediatric myocardial recovery.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Cardiomyocytes
- Cardiac repair and regeneration
- Engineered cardiac tissues
- Induced pluripotent stem cells
1 Introduction
Despite dramatic progress in the surgical management and survival of infants with complex congenital heart defects, myocardial injury following cardiac surgery and progressive cardiac dysfunction results in significant morbidity and mortality. There are currently many cardiac “cellular therapies” undergoing pre-clinical and clinical trials in adults. There have been small, encouraging clinical trials using stem cells in infants with hypoplastic left heart syndrome in Japan [1,2,3] that have led to similar trials in the United States [4,5,6]. It is clear that injected or implanted cells do not survive and that functional improvement occurs via paracrine mechanisms that impact angiogenesis and/or remodeling. Rapid advances in tissue engineering over the past two decades have resulted in the generation of functional, multicellular, 3D cardiac tissues with the potential for translation to human cardiac repair and regeneration [7,8,9,10,11,12,13,14]. This chapter provides a concise overview of some of the key issues in the generation, maturation, and translation of these engineered cardiac tissues (ECTs) and related work in our laboratory.
2 Continued Expansion of Engineered Cardiac Tissues (ECTs)
Following the initial description of 3D-reconstituted heart tissue using embryonic chick CM [7], CM species used to generate ECTs include chicken, mouse, and rat embryonic and post-natal CM, and a range of embryonic stem cells and induced pluripotent stem cells from rodents and human cell lines [8,9,10,11,12,13,14,15,16]. While initial formulations used a combination of cells, matrix factors, and collagen, additional compositions have utilized fibrin rather than collagen to reduce the effect of matrix stimulated inflammation [17] and tunable matrix compositions [18]. ECTs became increasingly popular as in vitro platforms for drug toxicology models [19] as well as for in vitro models for cardiac genetic disease modeling and repair [20].
3 Human Induced Pluripotent Stem Cell (H-iPSC) Linear ECTs
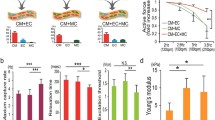
While cells from multiple vertebrate species have been used to investigate ECT structural and functional maturation and to model cardiomyopathies, it is clear that human cells are required for clinical trials and future therapies. H-iPSCs can be differentiated to cardiac lineages and produced in the quantities required for pre-clinical and future clinical trials. Multiple studies have validated the ability of h-iPSC-derived CM to mature and form implantable cell sheets [21]. To advance this paradigm, we developed and validated methods to generate linear (15 mm length × 1 mm diameter) ECTs from human iPSCs-derived CV lineages (h-iPSC-ECTs) [11]. We found that the coexistence of EC and MC vascular lineages with CMs within the 3D ECT compositions promoted tissue maturation (Fig. 54.1). Furthermore, we demonstrated the therapeutic potential of h-iPSC ECTs in an immune-tolerant rat myocardial infarction (MI) model showing the improvement of cardiac function with regenerated myocardium and enhanced angiogenesis [11].
H-iPSC linear ECTs generate functional cardiac tissues that recover cardiac structure and function following injury. (A) Schematic diagrams for three ECT compositions (upper) containing cardiomyocytes (CM), endothelial cells (EC), and/or mural cells (MC) and the proportions of each cell type used to generate ECTs (lower). (B) CM + EC + MC composition best preserved active force at increasing pacing rates. (C) Schematic timeline of rat MI surgery and ECT implantation (upper panel), three hiPSC-ECTs bundled for implantation (middle panel), and representative M-mode images for sham-operated (left) and ECT-implanted (right) rats 4 weeks after surgery (lower panel). (D) (a–c) Left ventricular (LV) histology 4 weeks after implantation of h-iPSC ECTs with Masson’s trichrome staining (upper left panel, 500 μm scale bar), double immunostaining for cTnT and HNA (human cell marker) with arrows indicating engrafted myocardium (upper right panel, 500 μm scale bar): (i) lower magnification with white arrows indicating capillary formation around grafted tissue; orange arrows indicate penetrating vasculature and white dotted line indicates vasculature within the ECT graft (200 μm) with higher magnification in (ii) 50 μm scale bar and (iii) 20 μm scale bar. (Adapted from [11])
4 Generation and Characterization of Large Format H-iPSC ECTs
Larger-format ECTs have been described using pre-vascularization, stacking cell sheets, scalable scaffolds, and bioprinting [12, 22]. Building on our success with linear h-iPSC ECTs and guided by initial works from the Bursac lab using polydimethylsiloxane (PDMS) molds, we fabricated a range of mold geometries from 0.5 mm thick PDMS sheets to generate a novel large-format hiPSC-ECT (LF-ECT, Fig. 54.2). As with our linear h-iPSC ECTs, these h-iPSC LF-ECTs undergo gel compaction, spontaneously and then synchronously beat, develop CM alignment along the internal bundle long axis, and survive in vivo implantation to recover myocardial function in an immune-tolerant rodent MI model [12]. Though unpublished, we have scaled this LF-ECT geometry to 30 × 30 mm, suitable for large animal pre-clinical studies.
H-iPSC large format LF-ECTs generate functional cardiac tissues that can recover cardiac structure and function following injury. (a) Schematic diagrams for three ECT compositions of LF-ECTs: ME-ECT with 7 mm posts in a pattern to generate a mesh, ML-ECT with 16 mm posts to generate multiple linear bundles, and PS-ECT to generate a central cell sheet (upper) and representative images after 14 days in vitro. (b) Prolonging in vitro culture from 14 days (blue) to 28 days (red) increased the rate of force generation and relaxation. Dashed lines highlight 90% contraction time (CT) and relaxation time (RT). (c) Representative Masson’s trichrome staining of SHAM and ME-ECT h-iPSC LF-ECT IMPLANT rat hearts 4 weeks post-implant. Red dotted line indicates engrafted area. Scale bar: 2 mm. (d) ME-ECT implantation reduced scar area (% of LV). (Adapted from [12])
5 Optogenetic Pacing of H-iPSC ECTs
One of the barriers to h-iPSC-derived ECT clinical translation is the relative electrophysiologic and contractile immaturity of h-iPSC-derived CMs. Strategies to mature CM within tissue-engineered structures include cyclic mechanical loading and electric field stimulation. As a novel approach, we designed proof-of-principle experiments to transfect h-iPSC-derived ECTs with a desensitization-resistant, chimeric channel rhodopsin, ChR (ChIEF) protein and then optically pace (OP) ECTs to accelerate maturation. We transfected h-iPSC ECTs using an AAV-packaged ChIEF and then verified OP by whole-cell patch clamp. ECTs were then chronically OP (C-OP) above their intrinsic beat rates in vitro from day 7 to 14. C-OP resulted in improved ECT electrophysiological properties and subtle changes in the expression of some cardiac relevant genes though active force generation and histology were unchanged (Fig. 54.3). These results validate the feasibility of a novel C-OP paradigm for non-invasive and scalable OP-induced ECT maturation strategies.
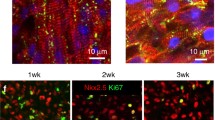
Optogenetic pacing (OP) of h-iPSC linear ECTs results in functional maturation. (a) Schematic for the generation of h-iPSC linear ECTs with CHiEF virus. (b) D14 chronic C-OP ECT showing 52% CM and 13% ChIEF-transfected cells. Nuclei (DAPI-blue), CM (cTnT-green), ChIEF-transfected cells with tdTomato (red). CM were predominantly located at the outer surface and the majority of transfected cells were CM. Inset in (b) shows higher magnification of double-positive cTnT and tdTomato cells. (c) Force measurement during intrinsic beating and during OP via 470 nm LED at 3.5 Hz. (d) Stress-frequency curves showed a less negative relationship after chronic (C-OP) or prolonged culture to D28 (unpublished data)
6 Incorporating Human Compatible Biomaterials into H-iPSC ECTs
An additional barrier to the clinical translation of h-iPSC-derived ECTs is the qualification of all reagents for human use as required by the FDA. Therefore, we revised our h-iPSC ECT formulation to replace rodent-derived Matrigel with human-derived and clinically acceptable MaxGel and replaced rodent type with human type I collagen. These experiments required multiple variations in composition but ultimately resulted in a human compatible h-iPSC-derived linear ECT with structural and functional features equivalent or better than ECTs formulated with rodent-derived matrix reagents (Fig. 54.4). While higher cost, these experiments provide additional insights for the formulations required for h-iPSC translation to humans.
Comparison of rodent-derived and human component-derived h-iPSC linear ECTs. (a) Representative images of rodent-derived (Matrigel and rat collagen I) and human compatible component (MaxGel and human collagen I) h-iPSC linear ECTs immediately after pouring on day 0 and after 14 days of in vitro culture. (b) Comparison of active stress on day 14 between 3 ECT compositions: Matrigel and rat collagen I, MaxGel and rat collagen I, and MaxGel and human collagen I. Active stress was greatest in human component ECTs (unpublished data)
7 Future Directions and Clinical Implications
Clearly, biological repair and replacement of lost myocardium using h-iPSCs that can form myocardium (versus skeletal muscle) are feasible, and clinical trials to determine safety and feasibility in adult heart failure patients should occur in the near future. It is important to note that many adult stem cell trials have shown statistically and clinically significant improvements in heart failure patients, and studies using stem cells have shown encouraging results for children with congenital heart disease [1,2,3,4]. The use of isolated cells, or the exosomes extracted from cells, may represent a much simpler therapeutic product to manufacture, verify, and distribute for future clinical therapy compared to the complexities of generating engineered tissues (multi-layer cell sheets and ECTs). Translation of these more complex formulations will require the engagement of biotechnology and biopharma companies with expertise in semi-automated manufacturing techniques and complex biologic quality controls not available in academic environments. Fortunately, every innovation in CV care for infants and children has been shown to also be valuable for the treatment of the much larger adult market. Therefore, the opportunities to translate h-iPSC ECTs towards infants and children with significant myocardial injury and heart failure remain encouraging.
References
Tsilimigras DI, Oikonomou EK, Moris D, et al. Stem cell therapy for congenital heart disease: a systematic review. Circulation. 2017;136(24):2373–85.
Sano T, Ousaka D, Goto T, et al. Impact of cardiac progenitor cells on heart failure and survival in single ventricle congenital heart disease. Circ Res. 2018;122:994. https://doi.org/10.1161/CIRCRESAHA.117.312311.
ClinicalTrials.gov. NCT01829750: Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease (PERSEUS). Phase I study to investigate the efficacy of intracoronary infusion of cardiac progenitor cells in patients with univentricular heart disease. Dr. Hidemasa Oh, PI.
Kaushal S, Wehman B, Pietris N, et al. Study design and rationale for ELPIS: a phase I/IIb randomized pilot study of allogeneic human mesenchymal stem cell injection in patients with hypoplastic left heart syndrome. Am Heart J. 2017;192:48–56.
ClinicalTrials.gov. NCT01883076: Autologous Umbilical Cord Blood Cells for HLHS. Phase I study to determine the safety and feasibility of injections of autologous umbilical cord blood (UCB) cells into the right ventricle of Hypoplastic Left Heart Syndrome (HLHS) children undergoing a scheduled Glenn surgical procedure. Dr. Tim Nelson, PI.
ClinicalTrials.gov. NCT02781922: Cardiac Stem/Progenitor Cell Infusion in Univentricular Physiology (APOLLON Trial). The purpose of this study is to evaluate the efficacy and safety of intracoronary injection of autologous cardiac stem cells (JRM-001) after reconstructive surgery in pediatric patients with functional single ventricle Japan Regenerative Medicine Co., Ltd, PI.
Eschenhagen T, Fink C, Remmers U, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11(8):683–94.
Tobita K, Liu LJ, Janczewski AM, et al. Engineered early embryonic cardiac tissue retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol. 2006;291(4):H1829–37.
Fujimoto KL, Clause KC, Liu LJ, et al. Engineered fetal cardiac graft preserves its cardiomyocyte proliferation within post-infarcted myocardium and sustains cardiac function. Tissue Eng Part A. 2011;17(5–6):585–96.
Tulloch NL, Muskheli V, Razumova MV, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59.
Masumoto H, Nakane T, Tinney JP et al 2016 The potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages for cardiac regeneration. Sci Rep 6:29933. YouTube: https://www.youtube.com/watch?v=dDUtxP-zcP0.
Nakane T, Masumoto H, Tinney JP, et al. Development of a large-format engineered cardiac tissue from human induced pluripotent stem cells-derived multiple lineage cardiac cells. Sci Rep. 2017;7:45641.
Kowalski WJ, Yuan FP, Nakane T, et al. Quantification of cardiomyocyte alignment from 3D confocal microscopy of engineered tissue. Microsc Microanal. 2017;23:826–42.
Fujita B, Zimmermann WH. Myocardial tissue engineering for regenerative applications. Curr Cardiol Rep. 2017;19(9):78. Review.
Tiburcy M, Hudson JE, Balfanz P, et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 2017;135(19):1832–47.
Li RA, Keung W, Cashman TJ, et al. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials. 2018;163:116–27.
Conradi L, Schmidt S, Neofytou E, et al. Immunobiology of fibrin-based engineered heart tissue. Stem Cells Transl Med. 2015;4(6):625–31.
Williams C, Budina E, Stoppel WL, et al. 3rd Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater. 2015;14:84–95.
Feric NT, Radisic M. Towards adult-like human engineered cardiac tissue: maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv Drug Deliv Rev. 2016;96:110–34.
Long C, Li H, Tiburcy M, Rodriguez-Caycedo C, et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci Adv. 2018;4(1):eaap9004.
Masumoto H, Ikuno T, Takeda M, et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2014;4:6716. https://doi.org/10.1038/srep06716.
Miyagawa S, Domae K, Yoshikawa Y, et al. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J Am Heart Assoc. 2017;6(4). pii: e003918) https://doi.org/10.1161/JAHA.116.003918.
Acknowledgments
This research was funded by the Kosair Charities Pediatric Heart Research Endowment Fund. All h-iPSCs used for this research were provided under a materials transfer agreement between the University of Louisville Research Foundation and the Center for iPS Cell Research and Application, Kyoto University. The authors acknowledge the collaborative support of Dr. Jun K. Yamashita, Principal Investigator, Department of Cell Growth and Differentiation, Center for iPS Cell Research and Application, Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Ye, F. et al. (2020). Progress in the Generation of Multiple Lineage Human-iPSC-Derived 3D-Engineered Cardiac Tissues for Cardiac Repair. In: Nakanishi, T., Baldwin, H., Fineman, J., Yamagishi, H. (eds) Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension. Springer, Singapore. https://doi.org/10.1007/978-981-15-1185-1_54
Download citation
DOI: https://doi.org/10.1007/978-981-15-1185-1_54
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1184-4
Online ISBN: 978-981-15-1185-1
eBook Packages: MedicineMedicine (R0)