Abstract
Substantial amounts of radionuclides including strontium-90 (90Sr) were released by the Fukushima Daiichi Nuclear Power Plant (FNPP) accident. In the present study, we describe and discuss the presence of 90Sr in the ex-evacuation zone of the FNPP accident and its relationship with 90Sr activity concentration in the hard tissue of animals. We found that the activity concentration of 90Sr in the hard tissue exhibited a positive correlation with 90Sr pollution in their corresponding terrestrial and marine environments. Hard tissues, such as the teeth, bones, and otoliths, of animals and fishes could serve as useful tools in assessing 90Sr pollution in the environment during the period of the formation of those tissues.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Strontium-90 ( 90Sr) has been released to the environment from various nuclear disasters in the past, such as nuclear weapon tests [1], the Chernobyl Nuclear Power Plant accident [2, 3], release of contaminated radionuclides from nuclear fuel processing plants at Sellafield and Mayak facilities [4, 5], and sea disposal operations by the former Soviet Union [6]. Strontium-90 was released also by the Fukushima Daiichi Nuclear Power Plant (FNPP) accident in 2011 and remained in the terrestrial and marine environments due to its long physical half-life of 28.8 years. Its long biological half-life and bone-seeking property may have adverse effects on the bone marrow, and special attention should be paid to the behavior of 90Sr. Strontium-90 easily transfers to terrestrial and marine biota because it is rather soluble [7, 8]. However, the relationship between 90Sr in the environment and in animal body is still unclear. In this chapter, we describe and discuss the presence of 90Sr in the environment and its migration into the teeth and bones of cattle abandoned in the ex-evacuation zone of the FNPP accident. We also discuss 90Sr activity concentration in the otolith of marine fishes around FNPP. Through the discussion, we suggest that 90Sr activity concentration in the hard tissue of animal body might reflect the extent of 90Sr pollution in the environment.
2 90Sr Pollution by the FNPP Accident
2.1 90Sr in the Environment
The Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT), reported that 0.1–6 kBq/m2 of 90Sr and 0.3–17 kBq/m2 of 89Sr were detected in soil within a 20-km radius from FNPP (the ex-evacuation zone) [9]. Strontium-90 is known to have been present in the Japanese environment before the FNPP accident, which probably stemmed from the atmospheric nuclear weapon tests conducted during the 1950s–1980s. However, the existence of 89Sr in soil of the ex-evacuation zone indicates that the 90Sr pollution was caused by the FNPP accident, because 89Sr has a relatively short half-life of 50.5 days and more than 50 years have passed since the atmospheric nuclear weapon tests were discontinued.
It was reported that the amount of 90Sr released by the FNPP accident into the atmosphere is smaller than those of volatile radionuclides (noble gases, iodine, tellurium, and cesium). The amount of released 90Sr is estimated to be three orders of magnitude smaller than that of cesium-137 (137Cs) [3]. The nuclear fuel of FNPP did not exceed the temperature of 2,700 K that is necessary for the volatilization of refractory elements, such as 90Sr and actinides [10]. Schwantes et al. estimated that most of radioactive Sr retained inside the reactors [11]. These reports suggest that the area polluted by 90Sr might be smaller than that by 137Cs attributed to the FNPP accident.
It was reported that 90Sr activity concentration in soil and vegetation samples is one to four orders of magnitude lower than that of 137Cs [12]. Sahoo et al. showed that 90Sr activity concentration in soil from the ex-evacuation zone of the FNPP accident ranged from 3 to 23 Bq/kg, whereas 137Cs activity concentration ranged from 0.7 to 110 kBq/kg [13], which is consistent with the previous reports [3, 10]. The ratio of 90Sr/137Cs in soil widely varies depending on the area examined.
The FNPP accident caused 90Sr pollution not only in the terrestrial environment but also in the marine environment. Since the most of 90Sr was presumed to remain in the reactor of FNPP, atmospheric release of 90Sr was speculated to be minor. The total amount of 90Sr released into the sea was estimated in the range from 0.09–0.9 PBq [14] to 1–6.5 PBq [15], and 90Sr activity concentration in surface seawater near the harbor of FNPP was 0.2–400 kBq/m3 [15]. The activity concentration of 90Sr was an order lower than that of 137Cs from April 2011 to February 2012, except for December 2011 when 90Sr and 137Cs activity concentrations were equivalent due to the discharge of 90Sr-contaminated wastewater [16]. In the Pacific Ocean 15 km east from FNPP, 90Sr activity concentration was two orders lower than that in seawater near FNPP. Owing to diffusion in offshore regions of Fukushima Prefecture, the pollution is believed to be limited to the sea around FNPP. These suggest that the 90Sr pollution of the terrestrial and marine environments are limited to the vicinity of FNPP.
2.2 90Sr Activity Concentration in the Hard Tissue of Abandoned Cattle After the FNPP Accident
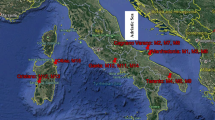
We previously reported activity concentration and specific activity of 90Sr in the teeth of cattle stayed in the ex-evacuation zone (Fig. 5.1) of the FNPP accident [17]. Because of chemical similarity between Sr and calcium (Ca), the teeth incorporate Sr during their formation period (calcification period) and retain it until they fall out or are worn down. As the amount of 90Sr accumulated in the teeth is assumed to reflect the amount of 90Sr incorporated into the body during the formation of the teeth, we hypothesized that the assessment of 90Sr activity concentration in the teeth might provide useful information about the degree of 90Sr contamination in the environment.
Figure 5.2 shows the relationship between 90Sr activity concentration in hard tissues (teeth and mandibular bones) of cattle and in soil from the ex-evacuation zone (Okuma Town and Kawauchi Village) after the FNPP accident. Activity concentrations of 90Sr in the teeth were 150–830 mBq/g Ca (ratio of 90Sr radioactivity to Ca weight in the tooth) (average, 470 mBq/g Ca) for Okuma Town and 100–310 mBq/g Ca (average, 170 mBq/g Ca) for Kawauchi Village [17]. The deposited amount of 90Sr in soil was 94–1500 Bq/m2 (average, 740 Bq/m2) for Okuma Town and 39–380 Bq/m2 (average, 200 Bq/m2) for Kawauchi Village [9]. We selected Iwate Prefecture as the control area because it is approximately 250 km north from FNPP and is considered free from FNPP-related 90Sr pollution. As shown in Fig. 5.2a, 90Sr activity concentration in cattle teeth was significantly correlated with that in soil; the higher the 90Sr activity concentration in soil, the higher the activity concentration in cattle teeth. A similar correlation was also found between 90Sr activity concentration in soil and that in the mandibular bone (Fig. 5.2b). Metabolic turnover rates (modeling and remodeling) of the mandibular bone in the cattle are not well-known. However, a major part of the original bone that formed before the FNNP accident was believed to be replaced by new bone formed after the accident, because, at the time the cattle were killed, 16–25 months had already passed since the accident. Thus, 90Sr activity concentrations in hard tissues (both in the teeth and bones) reflect environmental 90Sr levels during the formation of the tissues.
Relationship between 90Sr activity concentration in hard tissues and that in soil. (a) Relationship between teeth and soils. (b) Relationship between bones and soils. Data of 90Sr activity concentration in the teeth were obtained from our previous report [17]. Data of 90Sr activity concentration in soil were obtained from Japanese government reports [9, 18]. Each symbol indicates the average value with error bars showing the maximum and minimum values
2.3 90Sr Pollution in Fish
Miki et al. reported 90Sr activity concentrations in marine fish before and after the FNPP accident (Fig. 5.1) [19]. Activity concentrations higher than the background level before the FNPP accident were detected in 4 of 26 specimens collected just outside of the ex-evacuation zone, whereas 90Sr activity concentrations in all samples collected offshore the neighborhood of Fukushima Prefecture were under the detection limit. The report pointed out that 90Sr was detected only in marine fish living in the ocean near FNPP. Higher 137Cs activity concentration was found in the whole body of teleost fish; the activity ratio of 137Cs to 90Sr was 5–190 times higher than that before the accident.
Regarding 90Sr activity concentration in marine biota, the otolith of fish is a hard tissue composed of CaCO3 and might be a unique index of 90Sr pollution. In the process of otolith calcification, Sr is incorporated with Ca and is not metabolized once it is formed. It is reported that the β-ray count rate from otoliths of Japanese rockfish correlates with the activity concentration of radioactive Cs and 90Sr in the whole body of fishes in the main harbor of FNPP. On the other hand, no β-rays were detected from fish collected outside of the main harbor of FNPP [20].
The otolith could reflect 90Sr pollution in the living area of the fish. However, the use of otoliths as a biomonitor may not be feasible for detecting low levels of pollution, such as that outside FNPP harbor, because the amount of 90Sr would be too small in the otolith. If more sensitive determination methods, such as mass-spectrometric quantification [21], were developed, 90Sr in otoliths could be determined even with low radioactivity of the nuclide.
2.4 Migration of 90Sr from the Environment to Hard Tissues of Animals
Strontium in soil exists in two forms; one is an insoluble solid form, and the other is the soluble form (exchangeable fraction) that can be dissolved in water or other aqueous solutions such as ammonium acetate [22, 23]. Considering the migration of 90Sr from soil to hard tissues of animals, 90Sr in the latter form is believed to be important because Sr isotopes in the exchangeable fraction would be transferable in terrestrial biota. According to our analysis of soil from the ex-evacuation zone [23], only 1–9% of Sr in soil was in the exchangeable fraction, whereas 37–84% of 90Sr in soil was in the exchangeable fraction. Consequently, the specific activity of 90Sr (90Sr/total Sr ≈ stable Sr) in the solid form was low (23–33 Bq/g Sr in Kawauchi Village and 11–36 Bq/g Sr in Okuma Town), and specific activity in the exchangeable fraction was high (200–330 Bq/g Sr in Kawauchi Village and 130–1200 Bq/g Sr in Okuma Town). As both 90Sr and stable Sr behave similarly in the biota, the specific activity of 90Sr would basically be identical in the migration route of 90Sr from soil to plants and plants to animals (Fig. 5.3). In fact, specific activities of 90Sr in cattle teeth (260–640 Bq/g Sr in Kawauchi Village and 380–1400 Bq/g Sr in Okuma Town) were in a similar level as those in the exchangeable fraction in soil.
After the FNPP accident, cattle in the ex-evacuation zone were released to the polluted field and allowed to graze on contaminated grasses. Once it enters the body, Sr is known to be incorporated in hard tissues during their formation period and accumulate there. The main chemical form of Sr in the teeth and bones is hydroxyapatite, Ca10(PO4)6(OH)2, in which any fraction of Ca may be replaced by Sr with little change in the apatite structure. 90Sr incorporated in the hard tissue may exist as stable SrXCa(10-X)(PO4)6(OH)2 in the apatite crystal. Thus, 90Sr activity concentration in the hard tissue of animals reflects the environmental pollution level during the formation of the tissue. Soluble form of Sr may migrate through the food chain and get directly incorporated into fish body along with the absorption of Ca2+. 90Sr would be accumulated in the hard tissues of fish, such as the bones, teeth, and otoliths. Therefore, the hard tissue of fishes may also provide useful information about 90Sr pollution in the marine biota, as previously reported [20].
3 Summary and Perspectives for the Future Study
We described several aspects of 90Sr pollution in the environment after the FNPP accident and 90Sr activity concentration in the hard tissue of animal body. This article reviewed 90Sr pollution in soil and seawater, hard tissues of abandoned cattle, and contaminated marine fishes around FNPP after the accident. Although the degree of environmental pollution has gradually been declining for the last several years, continuous research is necessary for clarifying 90Sr mobility in the environment affected by the FNPP accident. Hard tissues such as the teeth, bones, and otoliths have the potential to reflect 90Sr pollution of the environment during their formation period. It should be emphasized that 90Sr specific activity in the teeth during their formation period and that in the exchangeable fraction in soil is at the same level [23]. This means that 90Sr specific activity in the teeth may serve as a useful tool for understanding the migration route of 90Sr in terrestrial biota. Furthermore, as the amount of 90Sr in the teeth may be proportional to the amount of 90Sr incorporated into the body during teeth formation, 90Sr in the teeth provides a useful index for the individual assessment of internal exposure to radiation. Estimation of individual exposure dose is essential for understanding the biological effect of radiation, especially animals affected by the FNPP accident. In this sense, the examination of radioactive nuclides including 90Sr in hard tissues provides useful information about the exposure dose to radiation. Nevertheless, further studies are required to elucidate the relationship between 90Sr in hard tissues and internal exposure dose to the nuclide.
References
United Nations Scientific Committee on the Effects of Atomic Radiation (2000) Sources and effects of ionizing radiation, vol I. UNSCEAR 2000 Rep. pp 193–291
Higley KA (2006) Environmental consequences of the Chernobyl accident and their remediation: twenty years of experience. International Atomic Energy Agency, Vienna. ISBN, p 166
Steinhauser G, Brandl A, Johnson TE (2014) Comparison of the Chernobyl and Fukushima nuclear accidents: a review of the environmental impacts. Sci Total Environ 470–471:800–817. https://doi.org/10.1016/j.scitotenv.2013.10.029
Smith JN, Ellis KM, Boyd T (1999) Circulation features in the central Arctic Ocean revealed by nuclear fuel reprocessing tracers from Scientific Ice Expeditions 1995 and 1996. J Geophys Res 104:29663. https://doi.org/10.1029/1999JC900244
Jones S (2008) Windscale and Kyshtym: a double anniversary. J Environ Radioact 99:1–6. https://doi.org/10.1016/j.jenvrad.2007.10.002
Yablokov AV (2001) Radioactive waste disposal in seas adjacent to the territory of the Russian Federation. Mar Pollut Bull 43:8–18. https://doi.org/10.1016/S0025-326X(01)00073-X
International Atomic Energy Agency (2004) Sediment distribution coefficients and concentration factors for biota in the marine environment. Technical Reports Series No. 422
International Atomic Energy Agency (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater. Technical Reports Series No. 472
Ministry of Education, Culture, Sports, Science and Technology, Japan (2011) Results of the nuclide analysis of Plutonium and Strontium by MEXT. Available at: http://radioactivity.nsr.go.jp/en/contents/5000/4167/24/1750_093014.pdf. Accessed 1 Sept 2015
Kirchner G, Bossew P, De CM (2012) Radioactivity from Fukushima Dai-ichi in air over Europe; part 2: what can it tell us about the accident? J Environ Radioact 114:35–40. https://doi.org/10.1016/j.jenvrad.2011.12.016
Schwantes JM, Orton CR, Clark RA (2012) Analysis of a nuclear accident: fission and activation product releases from the Fukushima Daiichi Nuclear Facility as remote indicators of source identification, extent of release, and state of damaged spent nuclear fuel. Environ Sci Technol 46:8621–8627. https://doi.org/10.1021/es300556m
Steinhauser G, Schauer V, Shozugawa K (2013) Concentration of strontium-90 at selected hot spots in Japan. PLoS One 8:1–5. https://doi.org/10.1371/journal.pone.0057760
Sahoo SK, Kavasi N, Sorimachi A et al (2016) Strontium-90 concentration in soil samples from the exclusion zone of the Fukushima Daiichi nuclear power plant. Sci Rep 6:23925. https://doi.org/10.1038/srep23925
Casacuberta N, Masqué P, Garcia-Orellana J et al (2013) 90Sr and 89Sr in seawater off Japan as a consequence of the Fukushima Dai-ichi nuclear accident. Biogeosciences 10:3649–3659. https://doi.org/10.5194/bg-10-3649-2013
Povinec PP, Gera M, Holý K et al (2013) Dispersion of Fukushima radionuclides in the global atmosphere and the ocean. Appl Radiat Isot 81:383–392. https://doi.org/10.1016/j.apradiso.2013.03.058
Tokyo Electric Power Co (2012) http://www.tepco.co.jp/cc/press/2012/1201877_1834.html. Accessed: 1th Sept 2018
Koarai K, Kino Y, Takahashi A et al (2016) 90Sr in teeth of cattle abandoned in evacuation zone: record of pollution from the Fukushima-Daiichi Nuclear Power Plant accident. Sci Rep 6:24077. https://doi.org/10.1038/srep24077
Ministry of Education, Culture, Sports, Science and Technology, Japan (2009) 51th bulletin of research results of environmental radioactivity. Available at: http://www.kankyo-hoshano.go.jp/08/ers_lib/ers_abs51.pdf. Accessed: 1th Sept 2018
Miki S, Fujimoto K, Shigenobu Y et al (2017) Concentrations of 90Sr and 137Cs/90Sr activity ratios in marine fishes after the Fukushima Dai-ichi Nuclear Power Plant accident. Fish Oceanogr 26:221–233. https://doi.org/10.1111/fog.12182
Fujimoto K, Miki S, Kaeriyama H et al (2015) Use of otolith for detecting strontium-90 in fish from the harbor of Fukushima Dai-ichi Nuclear Power Plant. Environ Sci Technol 49:7294–7301. https://doi.org/10.1021/es5051315
Takagai Y, Furukawa M, Kameo Y et al (2014) Sequential inductively coupled plasma quadrupole mass-spectrometric quantification of radioactive strontium-90 incorporating cascade separation steps for radioactive contamination rapid survey. Anal Methods 6:355–362. https://doi.org/10.1039/C3AY41067F
Krouglov SV, Kurinov AD, Alexakhin RM (1998) Chemical fractionation of 90Sr, 106Ru, 137Cs, and 144Ce in Chernobyl-contaminated soils: an evolution in the course of time. J Environ Radioact 38:59–76. https://doi.org/10.1016/S0265-931X(97)00022-2
Koarai K, Kino Y, Takahashi A et al (2018) 90Sr specific activity of teeth of abandoned cattle after the Fukushima accident – teeth as an indicator of environmental pollution. J Environ Radioact 183:1–6. https://doi.org/10.1016/j.jenvrad.2017.12.005
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Koarai, K. et al. (2020). Incorporation and Accumulation of Strontium-90 in the Hard Tissue of Animals and Their Relationship with Strontium-90 Pollution in the Environment. In: Fukumoto, M. (eds) Low-Dose Radiation Effects on Animals and Ecosystems. Springer, Singapore. https://doi.org/10.1007/978-981-13-8218-5_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-8218-5_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8217-8
Online ISBN: 978-981-13-8218-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)