Abstract
Amyloplasts, in which starch granules accumulate, are formed near the apical portion of sago palm stems. Amyloplast separation and division occur abundantly and specifically in the apical portion and in the basal stem during the middle and even in the late growth stage. Because of those separations and divisions, amyloplast sizes differ greatly among varieties and stem portions within a plant. The numbers of amyloplasts in the cross-sectional area of the parenchyma tissue also differ among cultivars. Generally, a stem parenchyma cell has 10–30 amyloplasts. Most amyloplasts are egg-like structures with a smooth surface. Still, the sago palm starch grain size is situated in the middle of grain sizes of 54 examined plant species. Furthermore, intercellular spaces are large in sago palm stem tissue, accounting for nearly 40–50% of their total space. This specific feature is a causal factor supporting the starch yield. These results suggest that the separation and division, amyloplast shape, amyloplast size diversity, and large intercellular spaces are specific to sago palm stems. Moreover, intercellular spaces are large in stem tissue, which might be a factor strongly affecting the starch yield.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
In higher plants, starch accumulation features are widely diverse among species, cultivars, and varieties, as are storage organs such as seeds, grains, roots, and stems. In the case of sago palm, great amounts of starch are accumulated in parenchyma cells in the stem (trunk). Within the stem, starch accumulates in cell organelles, called amyloplasts, in the center of ground parenchyma.
Many crops produce and accumulate starches. However, starch storage organs differ among crops: (A) grains are used by rice, wheat, corn (maize), barley, and other cereal crops; (B) tubers are used by potato, Jerusalem artichokes, and arrowroot; (C) tuberous root are used by sweet potato, cassava, dahlia, and snake gourd plants; and (D) other organs are used by lotus and banana plants.
Regarding starch accumulation in an amyloplast, if starch grains accumulate there, they are called simple starch grains such as sago palm stems, potato tubers, and yam tubers. If multiple starch grains accumulate in an amyloplast, the starch accumulation is called compound starch grains such as rice grains, taro tubers, and sweet potato tuberous roots.
For cereal crops, accumulation features of stored substances such as starches, proteins, and lipids have been investigated intensively, suggesting that starch accumulation features are widely diverse among species, cultivars, and varieties as well as growing area, culturing methods, and environmental conditions. Starch accumulation features of sago palm stem are also diverse.
As described in this paper, morphological and anatomical characteristics of starch accumulation features in sago palm stem are explained using a specific method and procedures with electron microscopy.
2 Research Methodology
Observation using scanning electron microscopy requires specific procedures because plant tissues, cells, and starches in parenchyma cells have an artifact structure during material preparation for observations and require charged viewing during observation. General preparation procedures for observation by scanning electron microscopy include the following: dissection of stem portions of small volumes (5 mm length × 5 mm width × 2 mm thickness) is preferred for materials. They are subjected to rapid freezing using slush nitrogen (−210 °C) followed by vacuum freeze-drying (−60 °C, 10−3 Pa) (Matsuda 2003). Subsequently, the revealed cross-sections were coated by OsO4 and/or platinum. In our laboratory, we use a scanning electron microscope (JSM6360A; JEOL, Japan). Cell and/or tissue cross-sectional areas are also measured using a personal computer with specific software (WinROOF; Mitani Corp., Japan).
This paper presents a summary of observation results obtained using several materials as follows.
-
(A)
Varieties Rotan, Tuni, and Molat were collected in Kendari, Southeast Sulawesi, Indonesia, in August 1999.
-
(B)
Plants of 2 years after trunk formation (YATF) were collected in Mukah, Sarawak, Malaysia, in July 2001.
-
(C)
Varieties were Wani (17–18 years YATF), Ruruna (20 YATF), Folo (20 YATF), Yepha Hongleu (18 YATF), Pane (20 YATF), Osukul (17–18 YATF), Para Hongleu (17–18 YATF), Rondo (12 YATF), Manno, Para Waliha, Yepha Hongleu, Rondo, Ruruna, and Para Hongleu. They were collected in the neighboring region of Lake Sentani, Papua Province, Indonesia.
-
(D)
Sucker. Collected from a four YATF plants in Mukah, Sarawak, Malaysia, in July 2008.
-
(E)
Stem. Collected at the Municipal Agriculture Training Center in Burauen, Leyte Province, Philippines, in July 2007.
Furthermore, potato tubers, Chinese yam tubers, wheat grains, edible canna tubers, rice grains, sweet potato tuberous roots, and eddoe tuberous roots were used derived from our laboratory collection.
3 Morphological and Anatomical Features of Sago Palm Stems and Starch
3.1 Internal Structure of Sago Palm Stem
In sago palm, starch is accumulated in parenchyma cells in the stem.
Monocotyledonous plants, like the sago palm, have no interfascicular cambium for secondary thickening and growth. Sago palm stem thickening depends solely on its primary growth and moreover has great ability and activity for thickening and growth from their apical portion to the base.
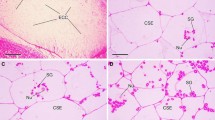
The sago palm stem encloses large intercellular spaces (Fig. 13.1) (Nakayama et al. 2007; Warashina et al. 2007). The percentage of intercellular spaces in the cross-sectional area of stem parenchyma is as great as 40–50%. Scanning electron microscope observation revealed that intercellular spaces are formed by surrounding six to eight parenchyma cells in the apical part of the stem (Fig. 13.1). A honeycomb structure produced by surrounding parenchyma cells was also observed, indicating the physical strengthening of the stem structure.
Scanning electron micrographs of parenchyma and intercellular spaces of sago palm stem: (a) apical meristem portion, (b) middle position of stem, P parenchyma, I intercellular space, Bar: 10 μm (Source: Nakayama et al. 2007)
3.2 Starch Accumulation Structure in Sago Palm Stem
In higher plants, starch granules accumulate in amyloplasts. Jong (1995) measured the long axes of amyloplasts in parenchyma cells of sago palm stem from after the trunk formation stage to the full trunk growth stage and the fruit ripening stage and reported that amyloplasts are longer at later stages of development. According to Ogita et al. (1996), amyloplasts within the stem parenchyma aged 3, 5, 8, and 13 years showed differences with increased age. They found that the major axis of the amyloplast was 5–20 μm in 3-year-old palms, 20–30 μm in 5-year-old palms, and greater than 30 μm in 8-year-old palms or 13-year-old palms. Kawasaki (1999) and Kawakami (1975) reported that when sago palm amyloplasts are compared with simple starch grains (simple starch grain type as described above) in other plants, they are larger than those in wheat (secondary starch granules, 2–8 μm diameter; primary starch granules, 20–40 μm diameter) or yam (about 20 μm diameter) but smaller than those in potato (10–90 μm diameter) or edible canna (40–100 μm diameter) (Table 13.1). In comparison with amyloplasts containing compound starch granules (compound starch grain type as described above), they are larger than those in rice (2.0–8.0 μm diameter), sweet potato (8.0–36.0 μm diameter), or taro (0.13–0.42 μm diameter). Jane et al. (1994) also reported from comparison of 54 crop species that sago palm amyloplasts are regarded as medium-sized among simple starch grain crop species.
3.3 Amyloplast Proliferation
Starch production of simple starch grain crops depends on the number and size of amyloplasts that contain starch. Proliferation of amyloplasts differs greatly among crops. In sago palm stems, the proliferation feature of amyloplasts is more specific than in other crops.
Nitta (2014) reported amyloplast proliferation of sago palm stems as follows (Fig. 13.2): a plastid, which is a precursor of an amyloplast, is formed near the apical portion. After commencement of starch accumulation, a plastid becomes an amyloplast. Amyloplasts are cell organelles after starch accumulation within a plastid.
Pattern of separation/division of sago palm amyloplast: (a) a protuberance is formed near the surface; (b) the basal portion of protuberance extends; (c, d), separation or division occurs in the middle portion of the major axis (c) or a little toward the protuberance from it (d); S stroma; ▲ protuberance (Source: Nitta 2014)
Amyloplasts in the early growth stage are mostly oval-shaped or spindle-shaped with a smooth, round surface. Many amyloplasts develop a protuberance in one part of the oval or spindle body at the middle stage of growth. In addition, a part of the oval or spindle has separated or is about to separate in many amyloplasts. The planes of separation are very flat and smooth.
Some amyloplasts separate in more than one place. No protuberance exists on the amyloplast surface after separation or immediately before separation in many cases.
In short, amyloplast proliferation of sago palm stems proceeds in the following manner (Fig. 13.2):
-
1.
Plastid accumulates starch (i.e., plastid becomes an amyloplast).
-
2.
A protuberance forms on the amyloplast surface.
-
3.
The base of the protuberance develops, and the protuberance grows out while the curvature of the oval or the spindle on the protuberance side becomes flatter.
-
4.
Separation or division occurs at the middle point along the major axis or on the protuberance side of the midpoint in the developed amyloplast.
These proliferation features, separation and division, occur in amyloplasts at or after the middle growth stage and in large amyloplasts at the early growth stage. In the protuberance, a stroma is apparently localized inside.
3.4 Amyloplast Size and Number Among Varieties
Mizuma et al. (2007) reported that the size and number of amyloplasts vary according to the position within the stem or by variety. Experiments using six sago palm varieties growing on the shores of Lake Sentani near Jayapura, Papua Province, Indonesia, close to the place of origin of sago palms, reveal that the major axis in all varieties is longer in amyloplasts in the middle and lower positions of the stem than in the upper positions. These results demonstrate that separation and division occur frequently in the middle and the lower positions of the stem. The major axis is short at the lower stem in Rondo and Para, suggesting that separation and division occur with high frequency on the basal side of the middle stem. The minor axis is also longer in the middle and lower positions than in the upper position in all varieties.
The numbers of amyloplasts in the cross-sectional area of the parenchyma tissue also differ among cultivars. Our experiments have found the most numerous amyloplasts in Rondo (262.4 mm−2) and the fewest in Para Wiliha (184.4 mm−2).
Starch accumulation features of potato tuber, Chinese yam tubers, wheat grains, and edible canna tubers are those of simple starch grain, while those of rice grains, sweet potato tuberous roots, and eddoe tuberous roots are those of compound starch grain. The starch major axis is longer in simple starch grains than in compound starch grains (Fig. 13.3).
However, considerable differences among varieties were observed in terms of the amyloplast size (Nitta et al. 2005, 2006, 2007). The major axis was longest in Pala Hongleu (38.7 μm) and shortest in Para Wiliha (27.7 μm).
In all varieties, the number of amyloplasts per unit cross-sectional area in the stem parenchyma is larger in the upper position than in the middle or basal position. Rondo and Yepha Hongleu, however, had more numerous amyloplasts in the lower position of the stem than in the middle, suggesting that separation and division occur frequently in these stem positions.
3.5 Sucker Structure and Starch Accumulation
In sago palm-growing areas such as Indonesia and Malaysia, transplanting is conducted using suckers for maintaining and propagating sago stubs. Suckers are formed in the basal portion of the mother stem accompanied by several emerging leaves. When the stem base diameter reaches about 10 cm, the sucker can be detached from the mother stem and transplanted into the field after brief nursery cultivation. Therefore, adequate management based on morphological and physiological characters is needed for rapid growth after transplantation.
In sago palm suckers, the cross-sectional area of parenchyma cells is larger in the lower stem portion. Intercellular spaces are formed even in the apical portion of the stem, followed by their enlargement in the lower stem portion. These features are almost identical to those of the main stem, as described above.
Plastid accumulates starch to become an amyloplast, followed by amyloplast enlargement. Amyloplasts, which are round and smooth, are formed in the apical meristem portion of the stem.
The occurrence of separation and division of amyloplasts is not often observed. Major and minor axes of amyloplasts are smaller in the upper stem portion. Arai et al. (2009) reported that suckers show not only early growth of parenchyma cells and tissues but also more rapid starch accumulation in amyloplasts than in the mother stem (Warashina et al. 2009) at the same distance from the apical meristem portion.
As described above, amyloplasts are formed in the apical meristem portion of sago suckers. They are round and smooth, with few occurrences of separation and division. These features differ from those of the mother stem.
3.6 Gelatinized Feature of Sago Palm Starch
One sago palm starch cooking method is heating in water. By heating in water, starches included in the sago palm swell and gelatinize. Gelatinized starch and non-gelatinized starch of commercialized sago palm starch after heating to 60 °C in water were assessed using scanning microscopic observation and comparison to starches of other species.
In gelatinized portions of sago palm starch, small holes of several micrometers in diameter are observed. Gelatinized starches are integrated into neighboring tissues. Holes of several micrometers in diameter and a thick skeleton structure are apparently causal factors of stickiness. Small pits (within several micrometers) were observed on the gelatinized starch surface.
Boiled sago palm starch, which is sold in city markets, revealed two distinct portions: a portion showing advanced gelatinization and a portion showing no advancement with remaining starch granules.
Stem materials of the Rondo cultivar are well known to be eaten after boiling in water in Indonesia. Scanning electron microscopic observations reveal that structures of vascular bundles, cells, and other tissues were not changed after boiling. However, boiling has advanced gelatinization not only within cells but also at the tissue level, including surrounding cells.
References
Arai Y, Nitta Y, Warashina S et al (2009) Internal structure and starch accumulation within the stem of sago sucker. In: Abstracts of the 227th meeting of the Crop Science Society of Japan. University of Tsukuba, Tsukuba, 27–28, March 2009
Jane J, Kasemsuwan T, Leas S et al (1994) Anthology of starch granule morphology by scanning electron microscopy. Starch 46:121–129
Jong FS (1995) Research of the development of sago palm (Metroxylon sagu Rottb.) cultivation in Sarawak, Malaysia. Ph. D. thesis, Agricultural University, Wageningen, The Netherlands
Kawakami I (1975) Starch morphology. Ishiyaku Publishers, Tokyo, pp 1–288
Kawasaki M (1999) Histological and cytological studies on synthesis and accumulation of storage substances in vegetative organs of tuberous crops. Ph. D. thesis, Tokyo University of Agriculture and Technology, Tokyo, pp 1–247
Matsuda T (2003) Morphological analysis on accumulation and mobilization of reserve substances in crops. In: Abstracts of the 215th meeting of the Crop Science Society of Japan. Chiba University, Chiba, 4–5, April 2003
Mizuma S, Nitta Y, Matsuda T et al (2007) Starch accumulation of sago palm grown around Lake Sentani, near Jayapura of the Papua province, Indonesia. In: Abstracts of the 223th meeting of the Crop Science Society of Japan. Ibaraki University, Ibaraki, 29–30 March 2007
Nakayama T, Nitta Y, Matsuda T (2007) Structure and function of intercellular spaces of sago palm (Metroxylon sagu Rottb.). In: Abstracts of the 223th meeting of the Crop Science Society of Japan. Ibaraki University, Ibaraki, 29–30 March 2007
Nitta Y (2014) Starch granule formation in parenchyma. In: The Society of Sago Palm Studies (ed) The sago palm the food and environmental challenges of the 21st century. Kyoto University Press, Kyoto, pp 210–216
Nitta Y, Warashina S, Matsuda T et al (2005) Varietal differences in amyloplast accumulation of sago palms grown around Lake Sentani near Jayapura, Indonesia electron microscopic study. In: The abstracts of the 14th conference of the Society of Sago Palm Studies. Tokyo University of Agriculture, Setagaya, Tokyo, 25 June 2005
Nitta Y, Mizuma S, Matsuda T et al (2006) Varietal differences of starch accumulation of sago palm grown around Lake Sentani, near Jayapura of the Papua province, Indonesia – electron microscopic study-. In: The abstracts of the 15th conference of the Society of Sago Palm Studies. Tohoku University, Sendai 24 June 2006
Nitta Y, Matsuda T, Mizuma S et al (2007) Diversity of starch accumulation in sago palm stem. In: Abstracts of the 9th international sago symposium – enhancing the potential of sago for food and industrial uses-. Sabin Resort Hotel, Ormoc, Philippines, 19–21 July 2007
Nitta Y, Asagi N, Homma T et al (2010) Morphological characters of sago palm starch. In: Abstracts of the 19th Conference of the Society of Sago Palm Studies. Ibaraki University, Ami, 19 June 2010
Ogita S, Kubo T, Takeuchi M et al (1996) Accumulations and distribution of starch in sago palm (Metroxylon sagu) stems. Sago Palm 4:1–5
Warashina S, Nitta Y, Matsuda T et al (2007) Formation of intercellular spaces and their function of sago palm stem. In: Abstracts of the 9th international sago symposium - enhancing the potential of sago for food and industrial uses-. Sabin Resort Hotel, Ormoc, Philippines, 19–21 July 2007
Warashina S, Nitta Y, Arai Y et al (2009) Morphological relations between thickening growth and structure of ground parenchyma tissue and/or starch accumulation of sago palm stem. In: Abstracts of the 227th meeting of the Crop Science Society of Japan. University of Tsukuba, Tsukuba, 27–28 March 2009
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Nitta, Y. (2018). Morphological and Anatomical Characteristics of Sago Palm Starch. In: Ehara, H., Toyoda, Y., Johnson, D. (eds) Sago Palm. Springer, Singapore. https://doi.org/10.1007/978-981-10-5269-9_13
Download citation
DOI: https://doi.org/10.1007/978-981-10-5269-9_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5268-2
Online ISBN: 978-981-10-5269-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)