Abstract
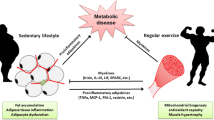
Growing evidence has shown that skeletal muscle cells can secrete bioactive proteins into the extracellular milieu. Secretion of many of those proteins is accelerated in response to exercise and muscle contraction and can regulate functions of several organs via autocrine, paracrine, and endocrine routes; this is referred to as the myokine theory. Habitual exercise leads to various health benefits such as metabolic improvement, anti-inflammation, and muscle building, which are at least partly caused by myokines including specific interleukins. Comprehensive analysis suggests that skeletal muscle cells can secrete over 100 proteins, many of which remain unknown. However, recent studies have identified additional novel myokines. Some secreted proteins can improve nutrient metabolism and muscle/bone mass; others exert anti-inflammatory and anti-tumorigenesis effects. The concept of myokines could be expanded to not only include high molecule weight proteins but also small peptides and noncoding RNA and applied to various fields including nutrition and medicine.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Baron AD, Brechtel G, Wallace P, Edelman SV (1998) Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Phys 255:E769–E774
Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B (2003) Searching for the exercise factor: is IL-6 a candidate. J Muscle Res Cell Motil 24:113–119
Burchfiel CM, Sharp DS, Curb JD, Rodriguez BL, Hwang LJ, Marcus EB, Yano K (1995) Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol 141:360–368
Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH (1992) A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 268:63–67
Lynch J, Helmrich SP, Lakka TA, Kaplan GA, Cohen RD, Salonen R, Salonen JT (1996) Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med 156:1307–1314
Gebel K, Ding D, Chey T, Stamatakis E, Brown WJ, Bauman AE (2015) Effect of moderate to vigorous physical activity on all-cause mortality in middle-aged and older Australians. JAMA Intern Med 175:970–977
Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S, JPHC Study Group (2007) Physical activity and risk of colorectal cancer in Japanese men and women: the Japan Public Health Center-based prospective study. Cancer Causes Control 18:199–209
Pedersen BK, Fischer CP (2007) Beneficial health effects of exercise--the role of IL-6 as a myokine. Trends Pharmacol Sci 28:152–156
Gusba JE, Wilson RJ, Robinson DL, Graham TE (2008) Interleukin-6 and its mRNA responses in exercise and recovery: relationship to muscle glycogen. Scand J Med Sci Sports 18:77–85
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33
Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, Krook A (2007) Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56:1630–1637
Benrick A, Wallenius V, Asterholm IW (2012) Interleukin-6 mediates exercise-induced increase in insulin sensitivity in mice. Exp Physiol 97:1224–1235
van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, MøllerK, Saltin B, Febbraio MA, Pedersen BK (2003) Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 88:3005–3010
Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, Pedersen BK (2005) Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro: evidence that IL-6 acts independently of lipolytic hormones. Am J Physiol Endocrinol Metab 288:E155–E162
Lyngso D, Simonsen L, Bulow J (2002) Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol 543:379–386
Lienenlüke B, Christ B (2007) Impact of interleukin-6 on the glucose metabolic capacity in rat liver. Histochem Cell Biol 128:371–377
Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY (2011) Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17:1481–1489
Matthews VB, Aström MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, AkerströmT, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA (2009) Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52:1409–1418
Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, ChibalinAV, Moller DE, Kharitonenkov A, Krook A (2011) Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev 27:286–297
Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M (2011) Upregulation of circulating IL-15 by treadmill running in healthy individuals: Is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J 58:211–215
Busquets S, Figueras M, Almendro V, López-Soriano FJ, Argilés JM (2006) Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochim Biophys Acta 1760:1613–1617
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, SanduskyGE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB (2005) FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635
Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, MangelsdorfDJ, Kliewer SA (2007) Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425
Lee MS, Choi SE, Ha ES, An SY, Kim TH, Han SJ, Kim HJ, Kim DJ, Kang Y, Lee KW (2012) Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-κB. Metabolism 61:1142–1451
Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK (2007) Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol 584:305–312
Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argilés JM (2009) Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab 296:E191–E202
Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T (2013) IL-15 overexpression promotes endurance, oxidative energy metabolism, and muscle PPARδ, SIRT1, PGC-1α, and PGC-1β expression in male mice. Endocrinology 154:232–245
Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK (2005) Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol 563:507–516
Pedersen L, Olsen CH, Pedersen BK, Hojman P (2012) Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. Am J Physiol Endocrinol Metab 302:E831–E840
Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW (2012) Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287:11968–11980
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16:1879–1886
Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, BellDR, Kralli A, Giacobino JP, Dériaz O (2003) Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52:2874–2881
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, SpiegelmanBM, Gerszten RE (2014) β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19:96–108
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157:1279–1291
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151:1319–1331
Adams GR (2002) Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol 93:1159–1167
Tahimic CG, Wang Y, Bikle DD (2013) Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne) 4:6
Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofibers hypertrophy in transgenic mice. J Biol Chem 270:12109–12116
Musaró A, McCullagh K, Paul A, Houghton L, Dobtowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200
Barton ER, Morris L, Musaró A, Rosenthal N, Sweeney HL (2002) Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 157:137–148
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL (1998) Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A 95:15603–15607
Li M, Li C, Parkhouse WS (2003) Age-related differences in the des IGF-I-mediated activation of Akt-1 and p 70 S6 K in mouse skeletal muscle. Mech Ageing Dev 124:771–778
Broholm C, Pedersen BK (2010) Leukaemia inhibitory factor – an exercise-induced myokine. Exerc Immunol Rev 16:77–85
Spangenburg EE, Booth FW (2002) Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Phys Cell Phys 283:C204–C211
Matsakas A, Diel P (2005) The growth factor myostatin, a key regulator in skeletal muscle growth and homeostasis. Int J Sports Med 26:83–89
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A 98:9306–9311
Allen DL, Unterman TG (2007) Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Phys Cell Phys 292:C188–C199
Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y (2002) The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277:40735–40741
Görgens SW, Raschke S, Holven KB, Jensen J, Eckardt K, Eckel J (2013) Regulation of follistatin-like protein 1 expression and secretion in primary human skeletal muscle cells. Arch Physiol Biochem 119:75–80
Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, EckelJ, Schürmann A, Eckardt K (2014) The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun 450:1089–1094
Görgens SW, Hjorth M, Eckardt K, Wichert S, Norheim F, Holen T, Lee S, Langleite T, Birkeland KI, Stadheim HK, Kolnes KJ, Tangen DS, Kolnes AJ, Jensen J, Drevon CA, Eckel J (2016) The exercise-regulated myokine chitinase-3-like protein 1 stimulates human myocyte proliferation. Acta Physiol (Oxford) 216:330–345
Haugen F, Norheim F, Lian H, Wensaas AJ, Dueland S, Berg O, Funderud A, Skålhegg BS, Raastad T, Drevon CA (2010) IL-7 is expressed and secreted by human skeletal muscle cells. Am J Phys Cell Phys 298:C807–C816
Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J (2013) Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem 288:1489–1499
Hamrick MW, McNeil PL, Patterson SL (2010) Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact 10:64–70
Jähn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, Bonewald LF (2012) Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater 24:197–209
Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S, Tang C, Wang Z, Yu J, Zhang G (2012) Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol 137:513–525
Hamrick MW (2012) The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. Bonekey Rep 1:60
Bakker AD, Kulkarni RN, Klein-Nulend J, Lems WF (2014) IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J Dent Res 93:394–399
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, NewMI, Zaidi M, Cinti S, Grano M (2015) The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A 112:12157–12162
Kaji H (2014) Interaction between Muscle and Bone. J Bone Metab 21:29–40
Yong Qiao X, Nie Y, Xian Ma Y, Chen Y, Cheng R, Yao Yinrg W, Hu Y, Ming Xu W, ZhiXL (2016) Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Report 6:18732
Forrester SJ, Kawata K, Lee H, Kim JS, Sebzda K, Butler T, Yingling VR, Park JY (2014) Bioinformatic identification of connective tissue growth factor as an osteogenic protein within skeletal muscle. Phys Rep 2:12
Covington JD, Tam CS, Bajpeyi S, Galgani JE, Noland RC, Smith SR, Redman LM, Ravussin E (2016) Myokine expression in muscle and myotubes in response to exercise stimulation. Med Sci Sports Exerc 48:384–390
Heinrich PC, Castell JV, Andus T (1990) Interleukin-6 and the acute phase response. Biochem J 265:621–636
Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285:E433–E437
Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK (2003) Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 17:884–886
Kadoglou NP, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M (2007) Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care 30:719–721
Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M (2008) Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc 56:2045–2052
Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K (2008) Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 283:32802–32811
Garabrant DH, Peters JM, Mack TM, Bernstein L (1984) Job activity and colon cancer risk. Am J Epidemiol 119:1005–1014
Zheng W, Shu XO, McLaughlin JK, Chow WH, Gao YT, Blot WJ (1993) Occupational physical activity and the incidence of cancer of the breast, corpus uteri, and ovary in Shanghai. Cancer 71:3620–3624
Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS (2001) Physical activity, obesity, height, and the risk of pancreatic cancer. J Am Med Assoc 286:921–929
Wannamethee SG, Shaper AG, Walker M (2001) Physical activity and risk of cancer in middle-aged men. Br J Cancer 85:1311–1316
World Cancer Research Fund, and American Institute for Cancer Research (2007) Physical activity. In: World Cancer Research Fund, American Institute for Cancer Research (ed) Food, nutrition, physical activity, and the prevention of cancer: a global perspective. WCRF/AICR, Washington, DC, pp 198–209
Hagio M, Matsumoto M, Yajima T, Hara H, Ishizuka S (2010) Voluntary wheel running exercise and dietary lactose concomitantly reduce proportion of secondary bile acids in rat feces. J Appl Physiol 109:663–668
Shephard RJ, Rhind S, Shek PN (1995) The impact of exercise on the immune system: NK cells, interleukins 1 and 2, and related responses. Exerc Sport Sci Rev 23:215–241
McTiernan A, Ulrich C, Slate S, Potter J (1998) Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control 9:487–509
Song BK, Cho KO, Jo Y, Oh JW, Kim YS (2012) Colon transit time according to physical activity level in adults. J Neurogastroenterol Motil 18:64–69
Demarzo MM, Martins LV, Fernandes CR, Herrero FA, Perez SE, Turatti A, Garcia SB (2008) Exercise reduces inflammation and cell proliferation in rat colon carcinogenesis. Med Sci Sports Exerc 40:618–621
Aoi W, Naito Y, Takagi T, Kokura S, Mizushima K, Takanami Y, Kawai Y, Tanimura Y, Hung LP, Koyama R, Ichikawa H, YoshikawaT (2010) Regular exercise reduces colon tumorigenesis associated with suppression of iNOS. Biochem Biophys Res Commun 399:14–19
Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, Koyama R, Wada S, Higashi A, Kokura S, Ichikawa H, YoshikawaT (2013) A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 62:882–889
Brekken RA, Sage EH (2001) SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 19:816–827
Bradshaw AD, Sage EH (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054
Bornstein P (1995) Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130:503–506
Jendraschak E, Sage EH (1996) Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol 7:139–146
Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD (2007) SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 282:22062–22071
Chlenski A, Guerrero LJ, Salwen HR, Yang Q, Tian Y, Morales La Madrid A, Mirzoeva S, Bouyer PG, Xu D, Walker M, Cohn SL (2011) Secreted protein acidic and rich in cysteine is a matrix scavenger chaperone. PLoS One 6:e23880
Nakamura K, Nakano S, Miyoshi T, Yamanouchi K, Matsuwaki T, Nishihara M (2012) Age-related resistance of skeletal muscle-derived progenitor cells to SPARC may explain a shift from myogenesis to adipogenesis. Aging (Albany NY) 4:40–48
Puolakkainen PA, Brekken RA, Muneer S, Sage EH (2004) Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res 2:215–224
Said N, Motamed K (2005) Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol 167:1739–1752
Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK, Berkowitz RS, Mok SC (2001) SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol 159:609–622
Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT (2008) SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2_deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer 98:1810–1819
Yang E, Kang HJ, Koh KH, Rhee H, Kim NK, Kim H (2007) Frequent inactivation of SPARC by promoter hypermethylation in colon cancers. Int J Cancer 121:567–755
Tai IT, Tang MJ (2008) SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Updat 11:231–246
Tai IT, Dai M, Owen DA, Chen LB (2005) Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest 115:1492–1402
Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K (2000) Altered expression of β-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis 21:1319–1327
Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK (2011) Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab 301:E504–E510
Bortoluzzi S, Scannapieco P, Cestaro A, Danieli GA, Schiaffino S (2006) Computational reconstruction of the human skeletal muscle secretome. Proteins 62:776–792
Chan XC, McDermott JC, Siu KW (2007) Identification of secreted proteins during skeletal muscle development. J Proteome Res 6:698–710
Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I (2010) Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics 9:2482–2496
Choi S, Liu X, Li P, Akimoto T, Lee SY, Zhang M, Yan Z (2005) Transcriptional profiling in mouse skeletal muscle following a single bout of voluntary running: evidence of increased cell proliferation. J Appl Physiol 99:2406–2415
Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA (2005) Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19:1498–1500
Guelfi KJ, Casey TM, Giles JJ, Fournier PA, Arthur PG (2006) A proteomic analysis of the acute effects of high-intensity exercise on skeletal muscle proteins in fasted rats. Clin Exp Pharmacol Physiol 33:952–957
Holloway KV, O’Gorman M, Woods P, Morton JP, Evans L, Cable NT, Goldspink DF, Burniston JG (2009) Proteomic investigation of changes in human vastus lateralis muscle in response to interval-exercise training. Proteomics 9:5155–5174
Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA (2007) Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21:2602–2612
van Hall G, Strømstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB (2009) Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29:1121–1129
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Aoi W, Sakuma K (2014) Does regulation of skeletal muscle function involve circulating microRNAs? Front Physiol 5:39
McCarthy JJ (2008) MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta 1779:682–691
McCarthy JJ (2011) The MyomiR network in skeletal muscle plasticity. Exerc Sci Sport Rev 39:150–154
Small EM, O’Rourke JR, Moresi V, Sutherland LB, McAnally J, Gerard RD, Richardson JA, Olson EN (2010) Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc Natl Acad Sci U S A 107:4218–4223
Aoi W (2015) Frontier impact of microRNAs in skeletal muscle research: a future perspective. Front Physiol 5:495
Acknowledgments
This work was supported by JSPS KAKENHI: Grant-in-Aid for Scientific Research (B) Grant Number 25282199, Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Aoi, W. (2017). Characteristics of Skeletal Muscle as a Secretory Organ. In: Sakuma, K. (eds) The Plasticity of Skeletal Muscle. Springer, Singapore. https://doi.org/10.1007/978-981-10-3292-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-3292-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3291-2
Online ISBN: 978-981-10-3292-9
eBook Packages: MedicineMedicine (R0)