Abstract
Periodontal ligament stem cells (PDLSCs) have been served as a cell reservoir for tissue regeneration during adulthood. For clinical applications, the challenging steps are to maintain the stem cell properties and to improve the regeneration capacity of PDLSCs during culture. Toll-like receptor 3 (TLR3) signaling has been shown to enhance therapeutic potential in several cell types including mesenchymal stem cells (MSCs) by inducing the secretion of multifunctional trophic factors. However, the role of TLR3 in PDLSCs is still unknown. The aim of this study was to investigate the responses of PDLSCs after TLR3 engagement using TLR3 agonist, poly(I:C). The result indicated that stimulation of TLR3 significantly enhanced pluripotent stem cell gene expression (e.g., REX-1 and SOX2) as well as immunomodulatory molecules (e.g., IFNγ and IDO). Interestingly, inhibition of NF-kB signaling decreased the TLR3-activated IFNγ but increased the TLR3-activated IDO expression, suggesting the multiple pathways in the inductive mechanism. Our finding supports the concept that activated TLR3 could encourage the stem cell and immunosuppressive properties of PDLSCs. Since immunosuppressive properties of stem cells could support tissue healing and regeneration, activation of TLR3 in PDL cells may trigger the effective PDL tissue regeneration.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Toll-like receptor 3 (TLR3) is a member of TLR family, the group of pattern recognition receptors (PRRs) that recognizes double-stranded RNA (dsRNA) produced by positive-strand RNA viruses and DNA viruses, resulting in pathogen clearance and recruitment of adaptive immune response [1]. TLR3-dsRNA interaction generally induces the expression and secretion of IFNγ and other proinflammatory cytokines through the Toll-interleukin-1 receptor domain-containing adaptor molecule-1 (TICAM-1, also known as TRIF) [2]. Recent study revealed that stimulation of TLR3 signaling by poly(I:C), the specific TLR3 agonist, enhanced immunosuppressive ability of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) through microRNA-143 inhibition [3]. Nonetheless, the therapeutic advantages of TLR3 activation are not only limited to its immunoregulatory response but also to its regenerative capacity by secretion of multifunctional trophic factors [4]. In bone marrow-derived MSCs (BM-MSCs), treatment with poly(I:C) potentially augmented the therapeutic capability for hamster heart failure through induction of relevant trophic factors such as interleukin-6 (IL-6), STO-1, hepatic growth factor (HGF), and vascular endothelial growth factor (VEGF) [5].
There is evidence suggesting that TLR3 may function as sensor to perceive the signal from damaged tissues upon injury. Literatures indicate that dsRNA released from damaged and necrotic cells can activate TLR3, resulting in an acceleration of the reepithelialization for wound closure. Moreover, dsRNA-induced TLR3 activation could promote the release of several cytokines including IL-6, IL-11, LIF, IL-10, SDF-1, VEGF, and HGF [6, 7]. However, the release of these cytokines is dependent on the type of cells and the concentration of ligand.
Periodontal ligament stem cells (PDLSCs) are the MSC-like cell population resided in periodontal ligament tissue, the connective tissue connected tooth root to the surrounding alveolar bone. Thus, PDLSCs have the potential to regenerate multiple cells comprising periodontal structures such as cementum, bone, and ligament and may pave the way for periodontal regenerative treatment via cell-based transplantation [8–10]. Apart from regenerative properties, the potential of PDLSCs for therapeutic application regarding to their immune privilege and immunosuppressive capability has been reported recently [11]. The immunomodulatory effect of DAMP-activated TLR3 has been indicated in periodontopathic gingival cells by stimulating TLR2-mediated inflammatory response to infected pathogen [12].
It has been proposed that for tissue regeneration, three interrelated events must be occurred. First, our body must sense the loss of tissue integrity, followed by the signals to induce the migration of precursor cells to reconstitute missing structures. Finally, these migrating cells must be signaled for an appropriate differentiation or functions [13]. However, upon infection or injury of PDLSCs, the signal that triggers PDLSC function for tissue regeneration has not been dignified yet. In this study, to imitate TLR3-dsRNA recognition, poly(I:C) was used for stimulating TLR3 in PDLSCs. The responses of PDLSCs after poly(I:C) treatment were investigated regarding to their stemness and immunosuppressive properties.
2 Materials and Methods
2.1 PDLSC Culture
Human PDLSCs were collected from healthy third molars as previously described [14]. The protocol was approved by the Ethical Committee, Faculty of Dentistry, Chulalongkorn University. PDLSCs were expanded in growth medium containing Dulbecco’s Modified Eagle’s Medium supplemented with 10 % fetal bovine serum, 2 mM L-Glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5 μg/ml amphotericin B. All cell culture reagents were purchased from Gibco BRL (Carls-bad, CA). Human PDLSCs were characterized for MSC properties as our previous report [15].
For osteogenic differentiation, PDLSCs were cultured in osteogenic medium which was normal growth medium supplemented with 50 μg/ml ascorbic acid, 100 nM dexamethasone, and 10 mM β-glycerophosphate.
2.2 Poly(I:C) Treatment
For TLR3 activation, PDLSCs were seeded at a density of 1.2 × 105 cells/well in 12-well plate in growth medium for 24 h and then treated with poly(I:C) at concentration of 5, 10 and 25 μg/ml for 24 h (InvivoGen, San Diego, CA) in fresh growth medium. PDLSCs cultured in medium without poly(I:C) were used as control.
2.3 Mineralization Assay
Detection of calcium deposition was evaluated on day 14 after cultured in osteogenic medium using the Alizarin red S staining assay. Briefly, cells were washed with phosphate-buffered saline (PBS) prior to being fixed with cold methanol for 10 min and stained with 1 % Alizarin red S staining solution (Sigma-Aldrich, St. Louis, MO).
2.4 Gene Expression Analysis
Total amount of RNA was extracted using Trizol reagent and 1 μg of RNA was converted into complementary DNA (cDNA) using a reverse transcriptase enzyme kit (Promega, Madison, WI). Conventional PCR was performed using Taq polymerase (Biotechrabbit Gmbh, Hennigsdorf, Germany) in a DNA thermal cycler (Biometra GmH, Göttingen, Germany). The products were electrophoresed on a 2 % agarose gel and visualized using ethidium bromide (EtBr; Bio-Rad, Hercules, CA).
qPCR was performed using SYBR GREEN FastStart Essential DNA Green Master which was detected by the LightCycler 96 (Roche Diagnostic GmbH, Mannheim, Germany). GAPDH was used as a reference gene for all experimental quantification. The primer sequences for qPCR can be reviewed in Table 17.1.
2.5 Flow Cytometry
PDLSCs (2 × 106 cells/sample) were dissociated and resuspended in 200 μl of FAC buffer and stained with MSC surface marker comprised of FITC-conjugated anti-human CD90 (Abcam, Cambridge, MA), PE-conjugated anti-human CD105 (BD Biosciences Pharmingen, San Diego, CA), and purified anti-human CD73 (Abcam). For CD73 detection, after incubating in primary antibody, cells were stained with biotinylated anti-mouse IgG2A followed by APC streptavidin. Stained cells will be analyzed on a FACSCalibur™ using the CellQuest™ software (BD Bioscience, San Jose, CA).
2.6 Statistical Analyses
Data were reported as Mean ± S.D. Statistical analyses of significance were evaluated using a two-tailed Student’s t-test or analysis of variance where values of P < 0.05 were considered significant. The analysis was performed by the statistical software (SPSS, Chicago, IL). A minimum of three replicates were analyzed for each experiment.
3 Results and Discussion
In periodontitis, the unhealthy cells of sulcular epithelium as well as gingival tissue were damaged either from the effect of proinflammatory cytokines or from mechanical stimulation such as chewing or brushing [16]. As in skin wound healing that was reported previously, the damaged or dying cells could release dsRNA, resulting in the activation of TLR3 and subsequently initiating tissue regeneration [17]. In this case, damaged cells in periodontitis may activate TLR3 in PDLSCs leading to the healing and regeneration of periodontal tissue.
4 TLR3 Activation Promote Stemness of PDLSCs
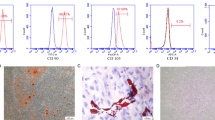
Human PDLSCs isolated from PDL tissue expressed the MSC markers including CD73, CD90 and CD105, as well as pluripotent markers such as REX-1, OCT4, NANOG, and SOX2. They also exhibited osteogenic characteristic as presented in upregulation of osteocalcin (OC) mRNA level and calcium deposition after osteogenic induction (Fig. 17.1). These results support that our isolated PDL cells possess stem cell characteristics, referred as PDLSCs.
Human PDL cells possessed the MSC characteristics. Human PDL cells were established from periodontal tissue obtained from extracted third molars. Cells from passage 3 were analyzed for the expression of mesenchymal stem cell markers. The results from FACS analysis showed that more than 90 % of cells expressed CD73, CD90, and CD105 (a). HPDL cells also expressed pluripotent markers such as Rex-1, Oct4, nanog, and Sox2 as judged by RT-PCR (b). For osteogenic differentiation ability, cells were cultured in osteogenic medium for 14 days. The increased expression of osteocalcin (OC) was detected. Moreover, in vitro calcification was observed as judged by alizarin red S staining (c). The figure was the representative results from three established PDL cell lines
Next, we examined the expressions of TLR2-4 in PDL tissue and PDLSCs. PDL tissue and dental pulp and gingival tissues from healthy patients were obtained with informed consent and subjected to RT-PCR analysis for the detection of TLR2-4 expressions. The results indicated that the expression levels of TLR2-4 were detected differently depended on cell types. Our study found that PDL tissue expressed the highest level of TLR3 compared to cells from dental pulp and gingiva. Moreover, gingival tissue showed the highest level of TLR2. No difference in expression of TLR4 was observed among tissue types (Fig. 17.2a).
Expression of TLR3 in periodontal tissue and PDL cells. Periodontal (PDL), dental pulp (pulp), and gingiva tissues were obtained from healthy patient and subject for RT-PCR analysis. The results (a) showed that all three tissues expressed TLR2, TLR3, and TLR4 with different levels. The highest expression of TLR3 was found in PDL tissue while gingiva expressed the highest level of TLR2. (b) showed the expression of TLR3 from three PDL cell lines (a–c). GAPDH was used as the internal control
As shown in Fig. 17.2, PDLSC itself promptly expressed TLR3. Poly(I:C) was used as specific TLR3 ligand to stimulate TLR3 function in PDLSCs. Cells were treated with poly(I:C) at concentration of 5, 10, and 25 μg/ml for 24 h. Our result showed the significant increased in mRNA levels of MSC-specific surface markers, CD73 and CD105, and the self-renewal markers REX-1 and SOX2 as compared to untreated control. No significant difference among poly(I:C)-treated groups was presented (Fig. 17.3a, b). Our result which corresponds with recent study indicated that activation of TLR3 by poly(I:C) promoted the cancer stem cell phenotype by upregulating the pluripotent genes including SOX2 in breast cancer [18]. Moreover, the increased mRNA level of hair progenitor-specific marker has been observed upon TLR3 activation in keratinocyte which contributed to hair follicle regeneration [17]. Attractively, TLR3 activation was required for induction of pluripotency in fibroblast by modification of epigenetic mechanism [19].
Poly(I:C) enhanced the expression of stem cell markers in human PDL cells. Human PDL cells were treated with poly(I:C) at concentration of 5, 10 and 25 μg/ml for 24 h. Real-time RT-PCR analysis showed that poly(I:C) enhanced the expression of MSC markers; CD73, CD105 (a), and pluripotent markers; Rex-1, Sox2 (b). * indicated the significant difference (p < 0.05). The results were shown as mean ± S.D. from three experiments
We imply that TLR3 may serve as a trigger receptor for damaged/infected response by enhancing the stemness of PDLSCs. Further determining of the signaling that involved in TLR3-acivated stem cell properties in PDLSCs would be needed to provide the better understanding of underlying mechanism of regeneration upon infection or injury. Besides, there are studies showing that the proliferation and differentiation capacities of PDLSCs had been weakened during in vitro culture expansion or in aged donors-derived, resulting in loss of proliferative and regenerative capacity [20, 21]. Here, we suggest that enhancement of PDLSC stemness by poly(I:C) treatment could concrete the way for PDLSC application in regenerative medicine.
5 TLR3 Activation Enhance Immunosuppressive Properties of PDLSCs
The MSC-like phenotypes of PDLSCs are not only the expression of stem cell markers, but also the immunosuppressive activity that can suppress immune responses after allogeneic transplantation [11]. The immune response interference of PDLSCs is generally processed through a cell-cell contact and secretion of immunomodulatory factors resulting in inhibition of T and B cell proliferation. Additionally, recent study indicated the immunosuppressive activity of PDLSCs is resisted even after osteogenic differentiation [22].
The role of TLR3 regarding the immunomodulation properties has been examined extensively in MSCs derived from several origins such as BM-MSCs [5], AD-MSCs [23], and UC-MSCs [3]; however, no comprehensive study in dental stem cells has been studied. In present study, after exposing to 25 μg/ml of poly(I:C) for 24 h, the upregulation of immunomodulatory mRNA levels, IFNγ, and IDO in PDLSCs was detected by qPCR (Fig. 17.4a). IFNγ is known as an upstream regulator of immunomodulatory molecules in MSCs. These cytokines promote an IDO production, resulting in restoring immunosuppressive ability of dental pulp stem cells (DPSCs) derived from irreversible pulpitis [24]. IDO is a catalytic enzyme of tryptophan, a crucial amino acid for T cell growth. Upregulation of IDO thus leads to the inhibition of T cell proliferation [25, 26]. In AD-MSCs, IDO is secreted in conditioned supernatant by IFNγ and potentially inhibited PBMC proliferation. Previous study indicated immunosuppressive mechanism of TLR3 in UC-MSCs through inhibiting microRNA-143 that results in IDO production [23]. We therefore denote that activation of TLR3 promotes immunosuppressive properties of PDLSCs via stimulating IFNγ and IDO. It has been shown that upon injury or inflammation of tissue, the immunosuppressive cytokines released from MSCs indicated to play a role in tissue homeostasis by inhibiting inflammatory response as well as stimulating the proliferation and differentiation of stem/progenitor cells [27]. By this cell protective mechanism, we prospect that activation of PDLSCs to secrete these immunosuppressive cytokines could augment the therapeutic potential of MSCs for tissue regeneration.
TLR3 activation induced immunosuppressive property of human PDL cells. Human PDL cells were treated with 25 μg/ml poly(I:C) for 24 h. RT-PCR analysis revealed that poly(I:C) significantly induced the expression of IFNγ and IDO (a). Addition of NF-kB inhibitor inhibited the poly(I:C)-induced IFNγ but could not inhibit the induction of IDO (b). Moreover, poly(I:C)-induced stem cell markers were also NF-kB independent (c, d). * indicated the significant difference (p < 0.05). The results were shown as mean ± S.D. from three experiments
6 TLR3 Activation Induces IFNγ Production in PDLSCs Via NF-kB Pathway
Normally, released dsRNA after viral infection is recognized by TLR3, and then activate NF-kB signaling, through TIR domain-containing adaptor TRIF [28, 29]. To examine whether activation of TLR3 in PDLSCs regulates production of IFNγ and its downstream IDO via NF-kB signaling, the NF-kB inhibitor was added into normal medium with and without poly(I:C) treatment. The mRNA expression of immunomodulatory molecules IFNγ and IDO was determined using qPCR. The result showed that, without poly(I:C) treatment, NF-kB inhibitor did not affect the endogenous IFNγ and IDO in PDLSCs. Interestingly, pretreatment of NF-kB inhibitor could attenuate the induction of IFNγ while further increasing IDO expression when compared to those in poly(I:C) treatment alone (Fig. 17.4b). Typically, IDO is induced by IFNγ in dendritic cells and macrophages. However, the regulation of IDO expression via IFNγ-independent pathways has been reported previously in astrocyte [30]. The result revealed that TLR3 activates IFNγ production in PDLSCs via NF-kB signaling, while TLR3-activated IDO expression may be possessed by other NF-kB-independent pathways.
Additionally, TLR3-activated NF-kB nuclear translocation has been reported to play an important role in epigenetic mechanisms during reprogramming of fibroblast into induced pluripotent stem cells (iPSCs) via retrovirus encoding reprogramming factors [19]. Therefore, it is possible that TLR3-induced stemness of PDLSCs was regulated via NF-kB signaling. To test this idea, the mRNA expression of pluripotent stem cell markers (e.g., REX-1 and SOX2) and MSC markers (e.g., CD73 and CD105) of TLR3-activated PDLSCs was determined after pretreatment with NF-kB inhibitor using qPCR. We found that addition of NF-kB inhibitor had no effect on the induction of those stem cell markers in either with poly(I:C) activation or NF-kB inhibitor alone in PDLSCs (Fig. 17.4c, d), though more studies are required for better understanding on how TLR3 signaling regulates stemness in PDLSCs.
7 Conclusion
In summary, our study discovered that TLR3 engagement by its specific ligand poly(I:C) could stimulate the stem cell properties of PDLSCs including self-renewal and immunomodulation. In periodontitis, it is possible that PDLSCs might generate new cells and prevent the immune cell-initiated inflammation for repairing the damaged structure through these mechanisms. Hence, TLR3 agonist may be a novel approach to enhance stem cell properties of PDLSCs for tissue regeneration, especially allogeneic cell-based therapy.
References
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413(6857):732–8.
O’Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors – redefining innate immunity. Nat Rev Immunol. 2013;13(6):453–60.
Zhao X, Liu D, Gong W, Zhao G, Liu L, Yang L, Hou Y. The toll-like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem Cells. 2014;32(2):521–33.
Delarosa O, Dalemans W, Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012;3:182.
Mastri M, Shah Z, McLaughlin T, Greene CJ, Baum L, Suzuki G, Lee T. Activation of toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency. Am J Physiol Cell Physiol. 2012;303(10):C1021–33.
Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol. 2004;172(11):6545–9.
Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, et al. Commensal bacteria regulate toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15(12):1377–82.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–55.
Gronthos S, Mrozik K, Shi S, Bartold PM. Ovine periodontal ligament stem cells: isolation, characterization, and differentiation potential. Calcif Tissue Int. 2006;79(5):310–7.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8(3):191–9.
Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219(3):667–76.
Mori K, Yanagita M, Hasegawa S, Kubota M, Yamashita M, Yamada S, Kitamura M, Murakami S. Necrosis-induced TLR3 activation promotes TLR2 expression in gingival cells. J Dent Res. 2015;94(8):1149–57.
Brockes JP, Kumar A, Velloso CP. Regeneration as an evolutionary variable. J Anat. 2001;199(Pt 1–2):3–11.
Pattamapun K, Tiranathanagul S, Yongchaitrakul T, Kuwatanasuchat J, Pavasant P. Activation of MMP-2 by Porphyromonas gingivalis in human periodontal ligament cells. J Periodontal Res. 2003;38(2):115–21.
Sawangmake C, Nowwarote N, Pavasant P, Chansiripornchai P, Osathanon T. A feasibility study of an in vitro differentiation potential toward insulin-producing cells by dental tissue-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2014;452(3):581–7.
Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64(1):57–80.
Nelson AM, Reddy SK, Ratliff TS, Hossain MZ, Katseff AS, Zhu AS, Chang E, Resnik SR, Page C, Kim D, et al. dsRNA released by tissue damage activates TLR3 to drive skin regeneration. Cell Stem Cell. 2015;17(2):139–51.
Jia D, Yang W, Li L, Liu H, Tan Y, Ooi S, Chi L, Filion LG, Figeys D, Wang L. beta-Catenin and NF-kappaB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ. 2015;22(2):298–310.
Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151(3):547–58.
Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675–82.
Sawa Y, Phillips A, Hollard J, Yoshida S, Braithwaite MW. The in vitro life-span of human periodontal ligament fibroblasts. Tissue Cell. 2000;32(2):163–70.
Tang R, Wei F, Wei L, Wang S, Ding G. Osteogenic differentiated periodontal ligament stem cells maintain their immunomodulatory capacity. J Tissue Eng Regen Med. 2014;8(3):226–32.
Lombardo E, DelaRosa O, Mancheno-Corvo P, Menta R, Ramirez C, Buscher D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 2009;15(7):1579–89.
Sonoda S, Yamaza H, Ma L, Tanaka Y, Tomoda E, Aijima R, Nonaka K, Kukita T, Shi S, Nishimura F, et al. Interferon-gamma improves impaired dentinogenic and immunosuppressive functions of irreversible pulpitis-derived human dental pulp stem cells. Sci Rep. 2016;6:19286.
Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–21.
Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806.
Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–16.
Kawai T, Akira S. Signaling to NF-kappaB by toll-like receptors. Trends Mol Med. 2007;13(11):460–9.
Sen GC, Sarkar SN. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 2005;16(1):1–14.
Suh HS, Zhao ML, Rivieccio M, Choi S, Connolly E, Zhao Y, Takikawa O, Brosnan CF, Lee SC. Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response. J Virol. 2007;81(18):9838–50.
Acknowledgments
This work was supported by Research Chair Grant 2012, the National Science and Technology Development Agency (NSTDA), Thailand. N.K. was supported by Grants for Development of New Faculty Staff, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University and the ASAHI Glass Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the work’s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work’s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2017 The Author(s)
About this paper
Cite this paper
Klincumhom, N., Chaikeawkaew, D., Adulheem, S., Pavasant, P. (2017). Activation of TLR3 Enhance Stemness and Immunomodulatory Properties of Periodontal Ligament Stem Cells (PDLSCs). In: Sasaki, K., Suzuki, O., Takahashi, N. (eds) Interface Oral Health Science 2016. Springer, Singapore. https://doi.org/10.1007/978-981-10-1560-1_17
Download citation
DOI: https://doi.org/10.1007/978-981-10-1560-1_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1559-5
Online ISBN: 978-981-10-1560-1
eBook Packages: MedicineMedicine (R0)