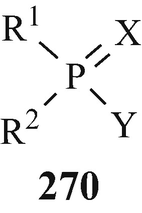Abstract
Ach (180) is the cationic neurotransmitter that in the central and peripheral nervous systems effects the transmission of action potentials across nerve-nerve and neuromuscular synapses. In response to an action potential it is released from the presynaptic nerve and then diffuses across the synapse ultimately to bind to the Ach receptor which serves, amongst other functions, as an ion gate for the entry of K⊕ into either the postsynaptic nerve process or the muscle or gland cell, the series of events that then follows ultimately resulting in the triggering of an action potential in the postsynaptic cell (Brand 1960; Gearien 1970; Greig et al. 1995a; Quinn 1987; Rosenberry 1975).
Notes
- 1.
Organophosphate Cholinesterase Inhibitors (including “nerve gases”)
The cholinesterase inhibitors of the organophosphate group, which have already been the subject of extensive review (Gaddum 1954; Holmstedt 1959; Marrs 1993; Silver 1974a; Soreq and Zakut 1993; Taylor 1996), can be denoted by the general formula 270 in which “R1 and R2 are capable of almost infinite variation. They may represent alcohols, phenols, mercaptans, amides, or alkyl or aryl groups attached directly to the phosphorus, etc. Common Y radicals are from fluorine, 4-nitrophenol and phosphates (in a “pyrophosphate”), but in other inhibitors Y may be cyanide, thiocyanate, an enol, a carboxylate, chloride or almost any phenoxy or thiophenoxy” (Holmstedt 1959) and “Y may contain a quaternary nitrogen atom and X may be either O or S” (Holmstedt 1959). Examples of this group of inhibitors containing these above structural functions in a variety of combinations have been presented in extensive (Holmstedt 1959) and less extensive (Silver 1974a; Taylor 1996) tabulations.
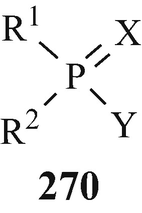
It has been stated that “The effects of organic phosphates on cholinesterase were discovered in various different ways. Tetraethylpyrophosphate (TEPP) which is one of the most active of these substances was first described as long ago as 1854 by de Clermont who said, among other things, that it had a burning taste. It has been unkindly said that the fact that de Clermont survived to tell the tale [a few drops should have been lethal (Taylor 1996)] makes it doubtful whether he really did succeed in making TEPP at all” (Gaddum 1954). The first published account of this group of substances (in the form of the esters of monofluorophosphoric acid) appeared in a paper by Lange and Krueger (1932) who accidently discovered that their odour was pleasant and aromatic but, a few minutes after inhalation, headache and breathlessness occurred followed by photophobia. The latter observations were apparently instrumental in the years beginning in the middle thirties, namely a few years before the outbreak of World War II, in leading a team led by Schrader at Farbenfabriken Bayer in Germany to investigate this class of compound for insecticidal activity. One of the early compounds from approximately 2,000 that were synthesised was Parathion (270, R1=R2=EtO, X=S, Y=4-NO2C6H4O) (Witkop 1998) that was to find use as an agricultural insecticide, in which role it also led to numerous cases of accidental poisoning. Another two compounds – which because of their extremely high toxicity in man were regarded as potential warfare agents and were therefore kept secret for several years – that were produced by Schrader’s group were Sarin (271) (Witkop 1998) [the structural analogue Soman (272) was also to prove to be an extremely toxic “nerve gas”] and Tabun (273) (Witkop 1998). Discoveries similar to those in Germany were made at the University of Cambridge by an extra-mural Ministry of Supply research team working under the direction of McCombie and Saunders during World War II and likewise – after a suggestion was put forward at the time of the Munich crisis in 1938, when war seemed imminent, for a study of the action of war gases on enzymes was not proceeded with – by a research team under the direction of Dixon working for the Chemical Defence Research Department of the Ministry of Supply in the Department of Biochemistry at Cambridge. For obvious security reasons, the results of these investigations were withheld from publication although secret reports – which were also made available to American workers almost from their inception – were from time to time submitted to the Ministry of Supply. However, from 1946 and onwards, following the cessation of hostilities, comprehensive summaries of this wartime research at Cambridge {which from the statement that “Until this work began in Cambridge in 1941, the alkyl fluorophosphates had received practically no attention. Lange [(Lange and Krueger 1932)] gave a tedious and laborious method for preparing dimethyl and diethyl fluorophosphates in very poor yields as follows.....”(McCombie and Saunders 1946) may appear to have received its genesis in the earlier German studies (vide supra)} have appeared (Dixon and Needham 1946; McCombie and Saunders 1946; Saunders 1947 – see also Henry 1949). Primarily it involved the synthesis and biological evaluation of alkyl fluorophosphates, the miotic action of which was noticed because of their effect on the eyes of those working with them, and ultimately lead to the discovery of the toxic effects of diisopropyl fluorophosphate (DFP) (270, R1=R2=Me2CHO, X=O, Y=F).
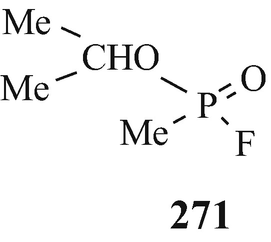
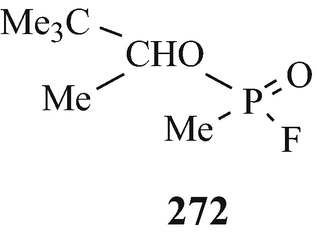
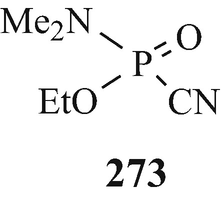
Thus, through the combined efforts in both Germany and the UK of the World War II machines were synthesised and biologically evaluated some of the most lethal of known synthetic compounds, namely Sarin (271), Soman (272), Tabun (273) and DFP (270, R1=R2=Me2CHO, X=O, Y=F), which thus became available for use as insidious chemical warfare “nerve gases”. What an appalling legacy for the world’s inhabitants!
‘Peace on earth!’ was said. We sing it,
And pay a million priests to bring it.
After two thousand years of Mass
We’ve got as far as poison gas.
[Thomas Hardy, Christmas: 1924 as quoted by Macinnis (2004) POISON AND WAR, Chapter 9. These sentiments were expressed when “gas-warfare” was limited to the use of the likes of chlorine, phosgene and mustard gas. However, they are currently certainly apposite in view of the incident during the protracted civil war in Syria where, during August 2013, a nerve gas was shamefully used against the civilian population of which a total of some thousands were either murdered or severely injured.]
Such is the nature of the human creature. However, through the more worthy and less destructive efforts of this same species, either l-physostigmine (Sect. 10.7.1) or its enantiomer (Sect. 10.9) have both been found to provide prophylactic protection against intoxication by organophosphates (including “nerve gases”) which is now known to occur via a progressive and virtually irreversible inactivation of AchE and other esterases by alkylphosphorylisation, involving in the former enzyme the hydroxyl group of the serinyl 198 moiety (Sect. 10.1.1 and Fig. 10.3) to afford 274 (Adrian et al. 1946; Porter et al. 1958; Rydon 1958; Silver 1974a; Taylor 1996; Witkop 1998). The slow rate of hydrolysis of the N-methylcarbamylated AchE (Fig. 10.1) is far surpassed by the almost irreversible combination of the so-called “nerve gases” as shown in 274 (Witkop 1998).
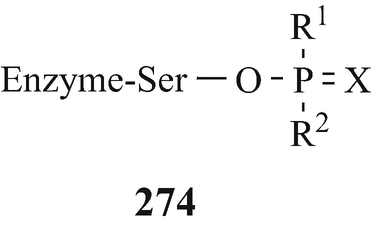
However, and fortunately in view of their widespread use, particularly in agriculture and horticulture and as insecticides, antidotes to the associated poisoning by organophosphates have been developed. These have been comprehensively reviewed (Holmstedt 1959; Silver 1974a; Taylor 1996) and consist of nucleophilic compounds, the most efficient being some of those with a strongly ionisable oxime (=NOH) group, which have a high affinity for phosphorus and thereby effect reactivation of the AchE by eliciting dephosphorylisation with liberation of the serinyl 198 hydroxyl group from the 274 moiety. Although not all oximes and hydroxamic acids are equally effective in this respect, they can also afford protection from poisoning if administered prior to exposure to the organophosphate (Holmstedt 1959) but in cases where this has already occurred, speedy treatment is essential since the phosphorylated enzyme “ages” to form the corresponding acid which is unable to react with the anionic oxime (Silver 1974a).
- 2.
It has been noted in review (Luo et al. 2006), that “human cholinesterases ....... contain up to 583 amino acids, have a molecular mass of 70-80 kDa and are variably glycosylated. Three-dimensional analyses of AChE and BChE, based on X-ray crystallography, have provided structural information regarding the positioning of the catalytically important amino acid residues within these proteins. In synopsis, three major binding domains have been described within AChE and two within BChE in an internalized, primarily hydrophobic gorge of some 20Å length but as narrow as 0.5Å wide. Deepest within this gorge is a catalytic acyl binding domain, which hydrolyses choline esters through electron transfer within a catalytic triad, termed a charge relay system. The triad includes Ser200, the imidazole of His440, and the carboxylic acid moiety of Glu327 (TcAchE numbering)..........”
- 3.
Whilst accepting that much of the early work on P.venenosum and the Calabar bean and its major alkaloidal component, l-physostigmine, was carried out in the University of Edinburgh (see footnote 8 of Chap. 1), this nevertheless contentious statement ignores the work of many others, such as, for examples, the Polonovski brothers – Max and Michel, and Robert Robinson and his co-workers, which contributed significantly to the structural elucidation of the alkaloid (Sect. 2.2) and the later pioneering studies of Julian and Pikl in the USA, of Kobayashi in Japan and of Robert Robinson in the UK, and their co-workers, which led to its first synthesis by Julian and Pikl (Sect. 2.3.1).
- 4.
In connection with these investigations, it has been stated that “The work of STEDMAN and co-workers stands as one of the high points in the structure-activity area and places them among the pioneers of this method of experimental approach which has yielded many active therapeutic agents in most branches of medicine” (Long 1963) and that they “were pioneers in chemical pharmacology” (Long and Evans 1967).
- 5.
For almost a century following its first isolation, l-physostigmine, and later together with l-physovenine (one of its alkaloidal companions in the Calabar bean) (Chap. 3), were the only alkaloids whose biological activities were associated with the inhibition of AchE (see footnote 8). Indeed, published opinion as to the occurrence of antiAchE activity within the alkaloid kingdom would appear to be divided. Thus, although the statement that “Alkaloids with strong cholinesterase inhibiting properties are a rare occurrence in the Plant Kingdom” (Holmstedt 1972) has been corroborated by “Alkaloids with strong anticholinesterase activity are very rare among plants” (Neuwinger 1996), it had earlier (Goldstein 1951) been pointed out that “The reversible inhibitors [of AchE] comprise a structurally diverse group whose only common feature is the presence of basic nitrogen [see also Sect. 10.1.1]. Indeed, it would appear that every alkaloid ever tested is an inhibitor at some concentration”. This assertation, which was accompanied by the observation that such activity is found with amphetamine, atropine, methylene blue, procaine, quinine hydrochloride and morphine [see also (Eadie 1941, Wright 1941)] and strychnine sulphates, has found support from the following reports:-
-
5.1.
Investigations by Beaujard (1944) and Vincent and Beaujard (1943, 1945) have led to the recognition of diverse alkaloidal antiAchEs. Perhaps not surprisingly, l-physostigmine was found to be by far the most active, geneserine was less active (see footnote 9) and in further decreasing order of activity were reported (Vincent and Beaujard 1943, 1945) pelletierine, ibogaine, conine (conicine), colchicine, strychnine, ergotinine, quinidine, papaverine, spartéine, quinine, emetine, cocaine, ergobasine, cinchonidine and cinchonine [only reported by (Vincent and Beaujard 1943)], thébaine, hyoscyamine, ergotamine [see also (Boyd et al. 1960)], versatrine, narcotine, narcéine, brucine, hordenine, dicodid [only reported by (Vincent and Beaujard 1943)], ephédrine, atropine, morphine (see also Eadie 1941; Vincent and Maugein 1942b; Wright 1941) dionine, yohimbine see also (Boyd et al. 1960) and apomorphine see also (Henry 1949), to be followed (Vincent and Beaujard 1943) by − in further decreasing order of activity − aconitine, mescaline, eucodal, codeine and heroine (Vincent and Maugein 1942b), cytisine, nicotine, pilocarpine, scopolamine and arécoline, – with the last four compounds being inactive, and (Vincent and Maugein 1942b) dihydroxicodienone.
-
5.2.
Later investigations (Raymond-Hamet et al. 1956) showed that akuammine (see also Creasey 1983(b)), harmaline, harmalol, harmine, ibogaine and quebrachamine (see also Creasey 1983(e)) “strongly inhibit the cholinesterase of horse serum and have a much weaker inhibiting action on the cholinesterase of sheep brain” – “in any case their action is weaker than that of physostigmine” and that (Levy-Appert-Collin 1969, 1978) pseudo-akuammigine is a more potent antiBchE than it is antiAchE. Yohimbine in high concentrations exhibits antiAchE activity (Creasey 1983(d); Boyd et al. 1960; Tanaka et al. 1978) as does ergotamine (Boyd et al. 1960).
-
5.3.
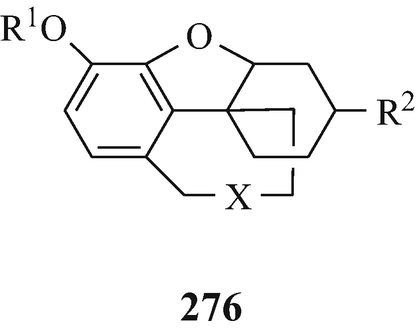
In 1952, galanthamine (275) was isolated from the Caucasian snowdrop, Galanthus woronowii Lozinsk (family Amaryllidaceae) (Holmstedt 1972; Wildman 1960), a plant native to Georgia in the USSR (Holmstedt 1972), and in the following year the same alkaloid, but now named lycoremine, was found in Lycoris radiata (Wildman 1960) and it has since been obtained “from the bulbs of a number of Amaryllidaceae plants” (Irwin and Smith 1960b) and “often is a constituent of the Galanthus, Leucojum, Narcissus and Vallota species” (Wildman 1960). Two other potential sources for it have also been suggested, both of which are snowdrops, namely Leucojum aestivum L. and Ungernia victoris Vved, which are often found on the Caucasian part of the Black Sea coast and grow in the southern parts of Central Asia, respectively (Holmstedt 1972). Catalytic hydrogenation of galanthamine afforded dihydrogalanthamine (276, R1=Me, R2=OH, X=NMe) which was found to be identical with lycoramine, an alkaloid that in 1932 had been isolated from Lycoris radiata (Wildman 1960), a subsequent botanical source of galanthamine (vide supra).
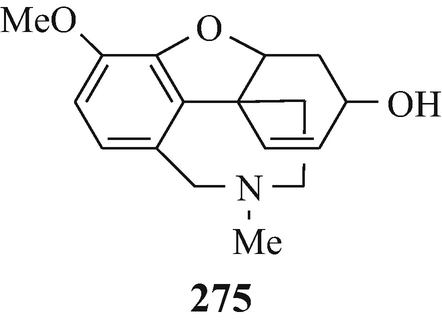
A century was to elapse from the discovery of that of l-physostigmine before another group of alkaloids with strong antiAchE activity was found (Holmstedt 1972) when galanthamine (275) was found to have potent antiAchE activity (Brossi et al. 1996; Holmstedt 1972), namely it is more active than pyridostigmine (189 R1=R2=Me) but less active than neostigmine bromide (188, R1=R2=Me, X=Br), and lycoramine (276, R1=Me, R2=OH, X=NMe) and is about equiactive with pyridostigmine (189 R1=R2=Me) (Irwin and Smith 1960a). As might have been expected, the methiodides of galanthamine and of deoxydemethyl lycoramine exhibit potent antiAchE activity (Irwin and Smith 1960b). Further structural modification, involving the introduction of a carbamyl function and quaternisation, afforded (276, R1=Me2NCO, R2=H, X=⊕NMe2IƟ) which is even more potent (Holmstedt 1972; Irwin and Hein 1962; Irwin et al. 1961). Galanthamine (275) has been used in the treatment of Mysasthenia gravis (Sect. 10.6.1) (Irwin and Smith 1960a) and other neurological diseases (Holmstedt 1972) and has been investigated for development for the treatment of Alzheimer’s disease (Sect. 10.7.2) (Brossi et al. 1996; Greig et al. 2005a; Klein 2007; Muñez-Ruiz et al. 2005; Thomsen et al. 1991).
-
5.4.
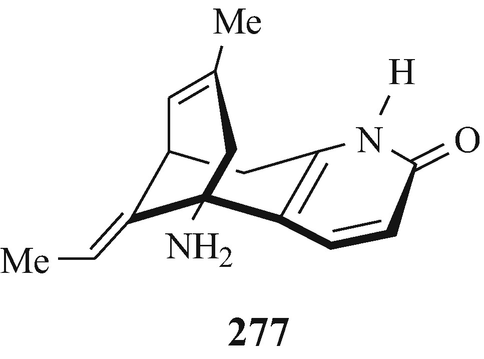
Huperzine (277), indigenous to China and isolated from the clubmoss Huperzia serrata, possesses antiAchE activity and is useful in the treatment of Myasthenia gravis (Chapter 10.6.1) and has been investigated for development for the treatment of Alzheimer’s disease (Sect. 10.7.2) (Brossi et al. 1996; Hanin et al. 1991).
-
5.1.
- 6.
Dedication
To the loving memory of my brother Roger and his wife Barbara. Together they introduced me to God’s own country of the North Yorkshire Moors National Park and many of its wonders. These, including especially the delightful village of Lastingham and its beautiful Ancient Crypt Church of St Mary (vide infra), we together shared with much joy and happiness on so many occasions.
Sydney Ringer (1835-1910) (Elliott 1914; Miller 2007)
The name of Sydney Ringer is perpetuated today in Ringer’s solution, the physiological saline which he invented during his work as a physiologist, pharmacologist and Professor of Medicine at University College, London. It is best known as the “drip” in evidence in hospital wards and operating rooms, and at accident sites but has also played a fundamental role – and continues to do so via pharmacological and physiological research using either modern cell culture media or either blood or tissue fluid replacement salines – in the advancement of medical science.
As well as his being primarily a physician in the University College Hospital, where one aspect of his research, as it had been that of others in 1869, 1871 and 1872 (Holmstedt 1972), involved the use of “Physostigma” in the treatment of nervous affections (Ringer and Murrell 1877), the “lectureship” in pharmacology in the college was “rendered illustrious by the name of Sydney Ringer” (Gaddum 1962). Upon his retirement, this ultimately led to the appointment of Arthur Cushny (1866-1926) [who played an important role in establishing pharmacology as a science and, as one of his main interests, was to effect important pioneering studies into biological relationships between optical isomers (Cushny 1926) (Sect. 10.9)] as the first Professor of Pharmacology in 1905 (Gaddum 1962). After thirteen years, Cushny moved to the inaugural chair in pharmacology in the School of Medicine at the University of Edinburgh (see footnote 8 of Chap. 1) where much of the early work upon Physostigma venenosum and l-physostigmine, the apparently (see Sect. 8.2) major alkaloidal component of its seeds (Calabar beans), was effected (Gaddum 1962) (see footnote 8, 9 and 10 of Chap. 1, Sects. 2.1, 2.2, and 10.1.1, 10.5). Included in this were the pioneering investigations into the physiological actions and therapeutic uses of the Calabar bean by TR Fraser (NOTE 20) whose appointment to the Chair of Materia Medica at Edinburgh had been “supported by a large number of famous people, including Sydney Ringer……” (Gaddum 1962) (see footnote 8 of Chap. 1).
However, as well as his contribution to medical practice and research, Sydney Ringer’s name is also synonymous with the Ancient Crypt Church of St Mary in the village of Lastingham (vide supra). This association transpired as a result of his purchase in Lastingham of a house, St Mary’s, which still stands, albeit now somewhat extended, in Anserdale Lane. Although this property eventually became his retirement home, throughout his late professional and earlier married life his principal home was 15 Cavendish Place, just off Regent Street and close to Harley and Wimpole Streets, Marylebone, W1 in London, and it was only for holidays and weekends that he visited his second home in Lastingham. Nevertheless, he became a leading member of the Parish, becoming a church-warden and a manager of the school – and in August 1867 he married Miss Ann Darley, the eldest child of Henry Brewster Darley, a Lord of the Manor of Lastingham.
The Ringers had two children, the eldest being Annie and the youngest being Hilda (née Sydney Ringer). Despite being availed of the then finest medical attention, Annie tragically died at the family home in London (vide supra) when she was only seven years old after a brief illness resulting from an intestinal obstruction. Her body was interred in the churchyard at Lastingham and her grave is now adjacent to that where her parents were later to find their final resting place. In memory of their daughter Annie, Sydney and Ann Ringer in 1879 became the benefactors of a substantial and major restoration of St Mary’s Church which was then in a very sorry state of disrepair, having been built beginning in 1078 upon the site of the Celtic monastery founded circa 654 by St Cedd from Lindisfarne who, following his death at Lastingham from plague, was succeeded as abbot by his brother St Chad. The result of this restoration is currently to be found in the wonderful Church of St Mary with its unique seventh century Saxon crypt – the location of the shrine of St Cedd – buried to the right of the altar, which has remained virtually unchanged since the time of William the Conqueror with the beautiful serenity of its celestial atmosphere. Perhaps not surprisingly, most of the stained glass windows in this church are Ringer memorials although, curiously, in several of these, Ringer’s Christian name is misspelled as Sidney (rather than the name associated with one of Australia’s major cities) [as it also is in (Elliott 1914) (vide infra)] and those of his wife and elder daughter as Anne.
Sydney Ringer died in 1910, having been predeceased by his wife Ann in 1897. They were survived by their second daughter, then Mrs Hilda Kayler, and it was she who, in 1912, endowed the Sydney Ringer Memorial Lecture to be delivered biennially at University College Hospital. As part of the inaugural presentation of this lecture in June 1914, it has been stated (Feldberg 1979) that TR Elliott (1914), by then an assistant physician to the hospital, “brilliantly anticipated and conceived the idea of chemical transmission in the autonomic nervous system”, although it has been noted (Bacq 1975) that it was a decade earlier that Elliott then had expressed “himself clearly on chemical transmission … .. In a short prophetic note presented to the Physiological Society on May 21, 1904” when he wrote “Adrenaline [the actual neurotransmitter was ultimately found to be Ach (180) (Sects. 10.1.1 and 10.3)] might then be the chemical stimulant liberated on each occasion when the impulse arrives at the periphery” (Elliott 1904) (Sect. 10.3).
- 7.
“With this two-fold experimental evidence the cholinergic nature of ganglionic and neuromuscular transmission was actually established although it took years before it was generally accepted. For the conversion of the strongest opponent, Eccles, we had to wait for over ten years” (Feldberg 1979). Thus, for example, it was stated in review (Henry 1949) that “It should be added that this essentially classical hypothesis does not tell the whole story of this physiological problem, and an interesting account of an electrical experiment of transmission has been given recently by Eccles [1945], and Cunliffe, Barnes and Beutner have called attention to the electrogenic properties of acetylcholine”. Who were these opponents?
They were the electrophysiologists who were in 1942 of the opinion that “It has now been established that neuromuscular transmission is mediated by the local negative potential which a nerve impulse sets up at the end plate region of a muscle fibre....Similarly, it has been shown that synaptic transmission may be mediated by the local negative potential” (Bacq 1975), a concept that had been further elaborated (Eccles 1945). Details of the evolution of this controversy over the years and of its ultimate solution have already been well and thoroughly presented (Bacq 1975; Eccles 1945; Feldberg 1979 – see also Sourkes 1966(ab)). In 1963, JC Eccles along with AL Hodgkin and AF Huxley were jointly awarded the Nobel Prize in Medicine and Physiology “For their discoveries concerning the ionic mechanisms involved in the excitation and inhibition in the peripheral and central portions of the nerve cell membrane” [Sourkes 1966(c)].
- 8.
It has been stated that “Although many alkaloids (Henry 1949) other than physostigmine have been isolated from the Calabar bean, apparently they have not been investigated for anticholinesterase (anti-ChE) activity” (Long 1963) and that “Although many alkaloids other than physostigmine have been isolated from the Calabar bean, they have not been evaluated for anticholinesterase or pharmacological activity” (Long and Evans 1967). However, it would appear that both of those related claims are at variance with the majority of the observations reported in the remainder of this NOTE and some of those reported in See footnote 9.
Included in the report (Salway 1911) announcing the first isolation from Calabar beans of l-physovenine (Sect. 3.1) [and the second isolation of l-eseramine ( 4.1)] was the observation that “Physovenine is, like physostigmine, very powerfully myotic [whereas no such activity whatsoever was ascribed to l-eseramine]; thus a single drop of 0.1 per cent solution of the alkaloid in dilute alcohol when introduced into the eye produced after an interval of a few minutes a powerful contraction of the pupil, which attained its maximum effect half an hour after the injection”. Moreover, others have noted that physovenine “a une action myotique beaucoup plus forte que l’ésérine” (Polonovski and Nitzberg 1915a) and has “a strong myotic action” [once again, a myotic action was not ascribed to l-eseramine] (Kerharo and Bouquet 1950) and, in broader context, “appears to be at least as poisonous as physostigmine” (Henry 1924).
These above observations have been further supported by the results from investigations (Ainscow et al. 1964) into the ability of l-eseramine and l-physovenine to potentiate the action of Ach on the frog rectus abdominus muscle (by the inhibition of AchE) and to reverse the effect of a tubocurarine block on the rat diaphragm-phrenic nerve preparation (by potentiation of the action of Ach). With both preparations l-physovenine shows the same order of activity as l-physostigmine, whereas the activity of l-eseramine is much lower (Ainscow et al. 1964). This latter observation was confirmed using Electric Eel AchE when it was also found that l-eseramine had only 0.7% of the potency of l-physostigmine (Yu et al. 1988a – see also Atack et al. 1989) – and, interestingly, also that all other analogues of physostigmine resulting from replacement of the methyl group at either N(2) (Atack et al. 1989, Yu et al. 1988a) or, surprisingly (see Sect. 10.7.2), within the carbamyl moiety (Atack et al. 1989) were only weak antiAchEs.
- 9.
It has been stated (Bacchi et al. 1994) that “geneserine inhibits acetylcholinesterase activity” although the presumably-supportive primary literature referred to (Brufani et al. 1986, 1987; de Sarno et al. 1989; Marta et al. 1988) unfortunately refers neither to such activity for geneserine nor even to the alkaloid whatsoever. However, from the results of several investigations (Bozonnet 1936; Kahane and Lévy 1936, 1937; Orzechowski and Hundreiser 1936; Polonovski et al. 1952; Vincent and Beaujard 1943, 1945; Vincent and Maugein 1942b; Vincent et al. 1961) it has been concluded (Robinson 1964b) that “geneserine shows anticholinesterase activity, but not to the same degree as does physostigmine” Indeed, it has also been concluded (Polonovski and Nitzberg 1915a) that, with regard to myotic action, geneserine is practically inactive and a summary (Henry 1924) of experimental observations (Polonovski and Combemale 1923) states that “Geneserine is not myotic and has generally a much weaker action than physostigmine and is less toxic”.
Pharmacological studies have also been carried out investigating the effect of l-geneserine upon the rabbit thyroid gland (Carrière et al. 1939), the guinea pig thyroid gland (Gineste and Parigot 1959), the endocrine function of the guinea pig pancreas (Gineste and Burin 1958), the inhibition of rat-brain monoamine oxidase (Vincent et al. 1961), gastric secretion in man (Filinski and Rostkowski 1927), neuromuscular excitability (Lobstein 1935) and salivary and pancreatic secretion (Polonovski and Combemale 1923).
- 10.
In 1854-1855, Robert Christison (see footnote 8 of Chap. 1) published the following cautionary observations with regard to the kernels of Calabar beans:-
“They are white and hard, but may be chewed; and they have the taste of the eatable leguminous seeds, without bitterness, acrimony, aroma, or any other impression on the organs of taste; in fact, they are scarcely, if at all, distinguishable in taste from a haricot-bean. This is a formidable peculiarity, were it possible for the seed to become a familiar poison in Europe.” (Christison 1854 – 1855, 1855).
It is, therefore, perhaps not surprising that cases of poisoning, either accidental or malicious, with the Calabar bean have been reported (Sect. 10.13).
References
Abramson SN, Radic Z, Manker D, Faulkner J, Taylor P (1989) Onchidal: a naturally occurring irreversible inhibitor of acetylcholinesterase with a novel mechanism of action. Mol Pharmacol 36:349–354
Adrian ED, Feldberg W, Kilby BA (1946) Inhibiting action of fluorophosphonates on cholinesterase. Nature 158:625
Aeschlimann JA, Reinert M (1931) The pharmacological action of some analogues of physostigmine. J Pharmacol Exp Ther 43:413–444
Ahmed M (1966) The synthesis of some potential antiacetylcholinesterases. MSc Thesis, Victoria Manchester
Ahmed M, Robinson B (1965) Synthesis of 1,3,3-trimethyl- and 1,2,3,3-tetramethyl-5-(methyl-and dimethyl-carbamoyloxy) indolines and their methiodides. J Pharm Pharmacol 17:728–733
Ainscow K, Ceasar PM, Cox B, Gill SE, Robinson B (1964) Unpublished results (1964) [quoted as ref 55 in (Robinson B 1968) and as ref 85 in (Ahmed 1966)]
Albuquerque EX, Deshpande SS, Kawabuchi M, Aracava Y, Idriss M, Rickett DL, Boyne AF (1985) Multiple actions of anticholinesterase agents on chemosensitive synapses: molecular basis for prophylaxis and treatment of organophosphate poisoning. Fundam Appl Toxicol 5:S181–S203
Andrews HL (1942) The effect of morphine and prostigmine methylsufate on measurements of pain threshold. J Am Med Assoc 120:525–527
Anon (1970) History of ophthalmology. Eserine and pilocarpine: our 100-years-old allies. In: Kronfeld PC (ed) Survey Ophthalmol, The Williams & Wilkins Co, 14 (6):479–485
Atack JR, Yu Q, Soncrant TT, Brossi A, Rapoport SI (1989) Comparative inhibitory effects of various physostigmine analogues against acetyl– and butyrylcholinesterases. J Pharmacol Exp Ther 249(1):194–202
Avison AWD (1954) Anticholinesterases. The relation between structure and activity of anticholinesterses containing quaternary-nitrogen groups. Chem Ind:288–293
Axelsson U (1969) Glaucoma miotic therapy and cataract. Acta Ophthalmologica Suppl 102:1–37
Axonyx (2005) (a) Axonyx to initiate phase 1 clinical trial of posiphen for Alzheimer’s disease; a Axonyx announces results of curtailed phase III clinical trials for phenserine in Alzheimer’s disease. Press release 1st August 1,2 and 20th September 1,2, respectively, and http://www axonyx com/pr/pr-20050801 html and 20050920 html, respectively; b Axonyx initiates second phase III Alzheimer’s disease trial with phenserine. Article date: 18th June 2004, and http://www.medicalnewstoday.commedicalnews php?newsid=9631 (20/10/2005) 1–3
Bacchi A, Pelizzi G, Redenti E, Delcanale M, Amari G, Ventura P (1994) Geneserine hydrochloride. Acta Cryst C50:1126–1130
Bacq ZM (1975) Chemical transmission of nerve impulses. A historical sketch. Pergamon Press, Oxford/New York/Toronto/Sydney/Braunschweig
Badger GM, Cook JW, Ongley PA (1950) The chemistry of the Mitragyna Genus. Part I. J Chem Soc 867–873 [see also refs 929,931,933,938 and 939 in (Hesse M 1964) but see (Finch N and Taylor WI 1963)]
Barger G (1936) From physostigmine to Prostigmin. Festschrift Emil C Barell, Basel, pp 7–17
Bartolini R, Aiello-Malmberg P, Bartolini A, Galli A, Renzi G (1978) 7th Int. congr. pharmacology, Paris, July 16th–21st, abstr 430 [quoted in (Galli et al. 1979)]
Bartolini A, Renzi G, Galli A, Aiello P, Bartolini R (1979) 1,2,3,3a,8,8a – Hexahydropyrrolo[2,3-b]indole derivatives. Ger Offen 2,839,279 [Chem Abs (1979) 91:5216p]
Bartolini A, Renzi G, Galli A, Malmberg-Aiello P, Bartolini R (1981) Eseroline: a new antinociceptive agent derived from physostigmine with opiate receptor agonist properties. Experimental in vivo and in vitro studies on cats and rodents. Neurosci Lett 25:179–183
Bartolini A, Renzi G, Galli A, Aiello P, Bartolini R (1982) 1,2,3,3a,8,8a – Hexahydropyrrolo[2,3-b]indole derivatives. Can CA 1,137,489 [Chem Abs (1983) 98:198532q]
Bartolucci C, Perola E, Cellai L, Brufani M, Lamba D (1999) “back door” opening implied by the crystal structure of a carbamoylated acetylcholinesterase. Biochemistry 38:5714–5719
Bartus RT, Dean IIIRL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Battersby AR, Openshaw HT (1953) The imidazole alkaloids. In: RHF M, Holmes HL (eds) The alkaloids chemistry and physiology. Academic, New York. 3: Chapter 20, pp 201–246
Beaujard P (1944) Recherches sur les alcaloïdes inhibiteurs de la cholinesterase. Thése Doct Pharm (Etat), Toulouse [quoted in (Vincent and Beaujard 1945)]
Becker R, Giacobini E (eds) (1991) Cholinergic basis for Alzheimer therapy. Birkhäuser, Boston/Basel/Berlin, pp 1–491
Bergmann F, Shimoni A (1952) Quaternary ammonium salts as inhibitors of acetylcholine esterase. Biochem Biophys Acta 8:520–525
Berry WK, Davies DR (1970) The use of carbamates and atropine in the protection of animals against poisoning by 1,2,2-trimethylpropylmethylphosphonofluoridate. Biochem Pharmacol 19:927–934
Birtley RDA, Roberts JB, Thomas BH, Wilson A (1966) Excretion and metabolism of [14C]-pyridostigmine in the rat. Br J Pharmacol 26:393–402
Blake Pritchard EA (1935) The use of “Prostigmine” in the treatment of Myasthenia gravis.Lancet Feb 23:432–434
Blockmans D, Steeno O (1988) Physostigmine as a treatment for anejaculation with paraplegic men. Physostigmin als behandlung bei anejakulation infolge paraplegie. Andrologia 20(4):311–313
Blow DM, Birktoft JJ, Hartley BS (1969) Role of a buried acid group in the mechanism of action of chymotrypsin. Nature 221:337–340
Boyd H, Chang V, Rand MJ (1960) The anticholinesterase activity or some antiadrenaline agents. Br J Pharmacol Chemotherapy 15:525–531
Boyer AW, Somani SM (1985) Pharmacokinetics of biliary excretaion of physostigmine in rat. Arch Int Pharmacodyn 278:180–192
Bozonnet EJ (1936) Inhibition of the hydrolysis of butyrylcholine by serum in the presence of geneserine. C R Soc Biol 123:920–922. [Chem Abs (1937) 31:18307 (not 8307 as recorded in the “author index”)]
Brand ED (1960) Neuropharmacology. In: Burger A (ed) Medicinal chemistry, 2nd edn, chapter 11. Interscience Publishers Inc., New York
Brindley GS (1984) The fertility of men with spinal injuries. Paraplegia 22:337–348
Brossi A (1985) Further explorations of unnatural alkaloids. J Nat Prod 48:878–893
Brossi A (1990) Physostigmine. In: Bioactive alkaloids. 4. Results of recent investigations with colchicine and physostigmine. J Med Chem 33: 2311–2319
Brossi A (1992) Fifteen years of research of bioactive alkaloids. Med Res Rev 12(1):1–26
Brossi A (1994) Chiral drugs:synopsis. Med Res Rev 14:655–691
Brossi A (1997) Alkaloids in medicine in encyclopedia of human biology, vol 1. 2nd edn. Academic,pp 243–247
Brossi A (2004) Personal communication
Brossi A, Pei X (1998) Biological activity of unnatural alkaloid enantiomers. In: Cordell GA (ed) The alkaloids chemistry and biology. Academic, San Diego/London/Boston/New York/Sydney/Tokyo/Toronto. 50: chapter 3, 109–139 and refs therein cited
Brossi A, Yu Q (1988a) Preparation of carbamates related to (+)-physostigmine as cholinergic agents.US Pat Appl US 166825 15 Jul 1988, Appl 04 Mar 1988; 21pp Avail NTIS Order no PAT-APPL-7-166 825 [Chem Abstr (1988) 109: 190632x]
Brossi A, Yu Q (1988b) Preparations of carbamates related to (−)-physostigmine as cholinergic agents. US Pat Appl US166824 15 Jul 1988, Appl 04 Mar 1988; 22pp Avail NTIS Order no PAT-APPL-7-166 824 [Chem Abs (1988) 109:190633y]
Brossi A, Schönenberger B, Clark OE, Ray R (1986) Inhibition of acetylcholinesterase from electric eel by (−)-and (+)- physostigmine and related compounds. FEBS Lett 201:190–192
Brossi A, Pei X, Greig NH (1996) Invited review phenserine, a novel anticholinesterase related to physostigmine: total synthesis and biological properties. Aust J Chem 49:171–181
Brown JH, Taylor P (1996) Muscarinic receptor agonists and antagonists. In: (Gilman et al. 1996) Chapter 7, 141–160
Brufani M, Filocano L (2000) Rational design of cholinesterase inhibitors. In: Giacobini E (ed) (2000)], Chapter 3, pp 27–45
Brufani M, Castellano C, Marta M, Oliverio A, Pavone F, Pomponi M (1985–1986) CNR Patent 47780 A84; U.S. Patent Appl n 705,009,1986; Japanese patent Appl n 38994/1985 [quoted as ref 5 in (Marta et al. 1988)]
Brufani M, Marta M, Pomponi M (1986) Anticholinesterase activity of a new carbamate, heptylphysostigmine, in view of its use in patients with Alzheimer-type dementia. Eur J Biochem 157:115–120
Brufani M, Castellano C, Marta M, Oliverio A, Pagella PG, Pavone F, Pomponi M, Rugarli PL (1987) A long-lasting cholinesterase inhibitor affecting neural and behavioral processes. Pharmacol Biochem Behav 26(3):625–629. [Chem abs (1987) 107:17658w]
Burks JS, Walker JE, Rumack BH, Ott JE (1974) Tricyclic antidepressants poisoning: reversal of coma, choreoatheosis, and myoclonus by physostigmine. J Am Med Assoc 230:1405–1407
Caine ED (1979) Anticholinergic toxicity. N Engl J Med 300:1278
Caltagirone C, Gainotti G, Masullo C (1982) Oral administration of chronic physostigmine does not improve cognitive or mnesic performances in Alzheimer’s presenile dementia. Int J Neurosci 16:247–249
Cameron J, Evans JH (1864) Report on the recent cases of poisoning by Calabar bean. Med Tms Gaz (London) 15:406–410
Camras CB, Siebold EC, Lustgarten JS, Serle JB, Frisch SC, Podos SM, Bito LZ (1989) Maintained reduction of intraocular pressure by prostaglandin F2-1- isopropyl ester applied in multiple doses in ocular hypertensive and glaucoma patients. Ophthalmol 96(9):1329–1337
Cannon JG (1981) Cholinergics .Chapter 43. In: Burger’s Medicinal Chemsitry, 4th edn, part III, Wolff ME (ed) Wiley, New York/Chichester/Brisbane/Toronto, pp 339–360 and refs therein cited
Cardenas DD, McLean A Jr, Farrell-Roberts L, Baker L, Brooke M, Haselkorn J (1994) Oral physostigmine and impaired memory in adults with brain injury. Brain Inj 8(7):579–587
Carrière G, Morel J, Gineste PJ (1939) Action of carbamoylcholine, geneserine and acetylcholine-geneserine mixture on the thyroid gland. C R Soc Biol 132:113–114. [Chem abs (1940) 34:8145
Casy AF (1970) Stereochemistry and biological activity. In: Burger A (ed) (1970), part I, chapter 7, pp 81–107
Casy AF, Parfitt RT (1986) Opioid analgesics chemistry and receptors. Plenum Press, New York/London, pp 69–73
Chapelle PA, Gaussel JJ, Grossiord A (1974) Réflexions concernant les problèms génito-sexuels des paraplégiques. Ann Med Phys 12:1–28
Chapelle PA, Blanquart F, Puech AJ, Held J-P (1983) Treatment of anejaculation in the total paraplegic by subcutaneous injection of physostigmine. Paraplegia 21:30–36
Chapelle PA, Raby-Brami A, Yakovleff A, Bussel B (1988) Neurological correlations of ejaculation and testicular size in men with a complete spinal cord section. J Neurol Neurosurg Psychiatry 51:197–202. [and also quoted in (Rawicki H and lording 1988)]
Chen YL, Nielsen J, Hedberg K, Dunaiskis A, Jones S, Russo L, Johnson J, Ives J, Liston D (1992) Synthesis, resolution, and structure-activity relationships of patent acetylcholinesterase inhibitors: 8-carbaphysostigmine analogues. J Med Chem 35:1429–1434
Christison R (1854–1855) On the properties of the ordeal-bean of Old Calabar, Western Africa. Pharm J Trans 14:470–476
Christison R (1855) On the properties of the ordeal-bean of Old Calabar, West Africa. Monthly J Med, 20 (3rd series, 11): 193–204 [for a somewhat abridged presentation of this article, see (Christison 1854–1855)]
Coleman BA, Michel L, Oswald R (1987) Interaction of a benzomorphan opiate with acetylcholinesterase and the nicotinic acetylcholine receptor. Mol Pharmacol 32:456–462
Cox B, Robinson B (1988) The reduction of 6-methoxy-9-methyl-11-oxoechiboline using lithium aluminium hydride. J Heter Chem 25:271–272
Coxworth E (1965) Alkaloids of the Calabar bean. In: Manske RHF (ed) The alkaloids chemistry and physiology, vol 8. Academic, New York/London, Chapter 2, pp 27–45
Creasey WA (1983) Pharmacology, biochemistry, and clinical applications of the monoterpenoid alkaloids, chapter 14. In: Saxton (ed) (1983), pp 783–829(a), 788(b), 789(c), 799(d), 806(e), 808–816(f), 816–819(g)
Cushny AR (1926) Biological relations of optically isomeric substances. Baillière, Tindall and Cox/London
Dale H (1958) Autobiographical sketch. Perspect Biol Med 1:125–137
Dale FJ (1969) The synthesis and anti-acetylcholinestease activity of (+)-physostigmine and (+)-physovenine. MSc Thesis, Victoria Manchester
Dale FJ, Robinson B (1970) The synthesis and anti-acetylcholinesterase activities of (+)-physostigmine and (+)-physoverine. J Pharm Pharmacol 22:889–896
Dalziel JM (1948) The useful plants of West Tropical Africa. The Crown Agents for the Colonies, 4.Millbank,Westminster, London, S.W.1 – under the authority of the Secretary of State for the Colonies, 256 [an appendix to the 1st edition of (Hutchinson and Dalziel 1958)]
Davidson M, Mohs RC, Hollander E, Davis M, Ryan R, Horvath TB, Davis KL (1986) Physostigmine in patients with Alzheimer’s disease. Psychopharmacol Bull 22:101–105
Davis KL, Mohs RC (1982) Enhancement of memory processes in Alzheimer’s disease with multiple-dose intravenous physostigmine. Am J Psychiatry 139:1421–1424
Davis KL, Mohs RC, Tinklenberg JR, Pfefferbaum A, Hallister LE, Kopell BS (1978) Physostigmine: improvement of long-term memory processes in normal humans. Science 201:272–274
Davis KL, Mohs RC, Tinklenberg JR (1979) Enhancement of memory by physostigmine. N Engl J Med 301:946
Davis KL, Mohs RC, Rosen WG, Greenwald BS, Levi MI, Horvath TB (1983) Memory enhancement with oral physostigmine in Alzheimer’s disease. N Engl J Med 308:721
Dayton HE, Garrett RL (1973) Production of analgesia by cholinergic drugs (37164). Proc Soc Exp Biol Med 142:1011–1013
de Sarno P, Pomponi M, Giacobini E, Tang XC, Williams E (1989) The effect of heptyl-physostigmine, a new cholinesterase inhibitor, on the central cholinergic system of the rat. Neurochem Res 14(10):971–977
Deshpande SS, Vianna GB, Kauffman FC, Rickett DL, Albuquerque EX (1986) Effectiveness of physostigmine as a pretreatment drug for protection of rats from organophosphate poisoning. Fundam Appl Toxicol 6:566–577
Deyi X, Linxiu W, Shuqiu P (1981) The inhibition and protection of cholinesterase by physostigmine and pyridostigmine against Soman poisoning in vivo. Fundam Appl Toxicol 1:217–221
Dirnhuber P, French MC, Green DM, Leadbeater L, Stratton JA (1979) The protection of primates against Soman poisoning by pretreatment with pyridostigmine. J Pharm Pharmacol 31:295–299
Dixon M, Needham DM (1946) Biochemical research on chemical warfare agents. Nature 158:432–438. (in particular “Alkyl fluorophosphonates” p 433)
Drachman DB (1994) Medical progress Myasthenia gravis. N Engl J Med 330:1797–1810
Drachman DA, Leavitt J (1974) Human memory and the cholinergic system a relationship to ageing. Arch Neurol 30:113–121
Drachman DA, Sahakian BJ (1980) Memory and cognitive function in the elderly. A preliminary trial of physostigmine. Arch Neurol 37:674–675
Dragstedt CA (1945) Trial by ordeal. Quart Bull Northwestern University Med School 19:137–141
Duvoisin RC, Katz R (1968) Reversal of central anticholinergic syndrome in man by physostigmine. J Am Med Assoc 206(9):1963–1965
Eadie GS (1941) The inhibition of cholinesterase by morphine in vitro. J Biol Chem 138:597–602
Eccles JC (1945) An electrical hypothesis of synaptic and neuromuscular transmission. Nature 156(3971):680–683
Edwards PN, Smith GF (1960) Akuamma alkaloids, part III. ψ- Akuammicine. Proc Chem Soc 215
Elliott TR (1904) On the action of adrenalin (Preliminary communication). In: Proceedings of the Physiological Society May 21. J Physiol Lond 31:20–21
Elliott TR (1905a) The action of adrenalin. J Physiol 32:401–467
Elliott TR (1905b) The action of adrenalin. Scientific grants committee of the British Medical Association. Report XCIII. Br Med J July 15:127–130
Elliott TR (1914) The Sidney [sic (NOTE 21)] ringer memorial lecture on the adrenal glands. Br Med J 1:1393–1397
Ellis S, Krayor O, Plachte FL (1943) Studies on physostigmine and related substances. III breakdown products of physostigmine; their inhibitory effect on cholinesterase and their pharmacological action. J Pharmacol 79:309–319
Faerman C, Ripoll D, Bon S, Feuvre YL, Morel N, Massoulié J, Sussman JL, Silman I (1996) Site-directed mutants designed to test back-door hypotheses of acetylcholinesterase function. FEBS Lett 386:65–71
Feldberg W (1968) Letter to the author, 26th April [quoted as ref 76 in (Holmstedt 1972)]
Feldberg W (1969) Henry Hallet Dale (1875–1968). Br J Pharmacol 35:1–9
Feldberg W (1979) The early history of synaptic and neuromuscular transmission by acetylcholine: reminiscences of an eye witness. In: The pursuit of nature (informal essays on the history of physiology). The Physiological Society, London, (Cambridge University Press), pp 65–83
Feldberg W, Fessard A (1942) The cholinergic nature of the nerves to the electric organ of the torpedo (Torpedo marmorata). J Physiol (Lond) 101:200–216 (note 1 on p 201)
Feldberg W, Gaddum JH (1934) The chemical transmitter at synapses in a sympathetic ganglion. J Physiol 81:305–319
Filinski W, Rostkowski C (1927) Influence of geneserine on the secretion of the gastric juice in man. CR Soc Biol 97:952–954. [Chem Abs (1928) 22:272–273]
Fisher MA (2002) Use of cholinesterase inhibitors in the therapy of Myasthenia gravis. In: [Giacobini E (ed) 2000a] Chapter 15, pp 249–262
Flippen-Anderson JL, Deschamps JR, Brossi A, Greig NH (2002) 2-Hydroxybenzoic acid salt of physostigmine. Acta Cryst E58:0853–0855
Flodmark S, Wramner T (1945) The analgetic action of morphine, eserine and prostigmine studied by modified hardy-Wolff-Goodell method. Acta Physiol Scand 9:88–96
Fraser TR (1862) On the characters, actions and therapeutical uses of the ordeal bean of Calabar. Inaugural MD dissertation for which a gold medal was awarded by the University of Edinburgh at the medical graduation of August 1862 [quoted in (Fraser TR 1863 and Rodin 1947) – see also ref 6 in (Barger 1936)]
Fraser TR (1863) On the characters, actions, and therapeutical uses of the ordeal bean of Calabar (Physostigma venenosum, Balfour). Edinburgh Med J 9. 36–56, 123–132, 235–248
Fraser FR (1938a) The clinical aspects of the transmission of the effects of nervous impulses by acetylcholine, lecture I. Br Med J 1:1249–1254
Fraser FR (1938b) The clinical aspects of the transmission of the effects of nervous impulses by acetylcholine, lecture II. Br Med J 1:1293–1299
Fraser FR (1938c) The clinical aspects of the transmission of the effects of nervous impulses by acetylcholine, lecture III. Br Med J 1:1349–1354
Fritz H, Stock E (1970) Synthese eines tetracyclischen α-aminoindolins mit den strukturmerkmalen deseserins über eine Fischersche indolsynthese mit fixierung des β-stickstoffs. Tetrahedron 26:5821–5829
Fühner H (1918) Der toxicologische nachweiss der physostigmine. Biochem Z (Berlin) 92:347–354. [quoted as ref 75 in (Holmstedt 1972)]
Fürst S, Friedmann T, Bartolini A, Bartolini R, Aiello-Malmberg P, Galli A, Somogyi GT, Knoll J (1982) Direct evidence that eseroline possesses morphine – like effects. Eur J Pharmacol 83:233–241
Gaddum JH (1954) Anticholinesterases. The history of work on anticholinesterases. Chem Ind:266–268
Gaddum JH (1962) The pharmacologists of Edinburgh. Ann Rev Pharmacol 2:1–11
Galli A, Renzi G, Bartolini R, Malmberg-Aiello P (1979) Inhibtion of [3H]naloxone binding in homogenates of rat brain by eseroline, a drug, with analgesic activity, related to physostigmine. J Pharm Pharmacol 31:784–786
Galli A, Renzi G, Grazzini E, Bartolini R, Aiello-Malmberg P, Bartolini A (1982) Reversible inhibition of acetylcholinesterase by eseroline, an opioid agonist structurally related to physostigmine (eserine) and morphine. Biochem Pharmacol 31:1233–1238
Gardner JH, Stevens JR (1947) Some urethans of phenolic quaternary ammonium salts. J Am Chem Soc 69:3086–3088
Gearien JE (1970) Cholinergics and anticholinesterases. In: Burger A (ed) (1970), part II, chapter 47, pp 1296–1313
Giacobini E (2000a) Cholinesterase inhibitors: from the Calabar bean to Alzheimer therapy. In: Giacobini E (ed) (2000), Chapter 12, pp 181–225
Giacobini E (ed) (2000b) Cholinesterases and cholinesterase inhibitors. Martin Dunitz, London
Giacobini E (2004) Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacol Res 50:433–440
Gilson MK, Straatsma TP, McCammon JA, Ripoll DR, Faerman CH, Axelsen PH, Silman I, Sussman JL (1994) Open “back-door” in a molecular dynamics simulation of acetylcholinesterase. Science 263:1276–1278
Gineste PJ, Burin P (1958) Action of anticholinesterases on the endocrine function of the pancreas of the guinea pig. CR Soc Biol 152:510–511. [Chem Abs (1959) 53:3477f]
Gineste PJ, Parigot C (1959) Action of geneserine, carbamoylcholine, acetyl-ß-methylcholine, and other related substances on the thyroid gland of the guinea pig. CR Soc Biol 153:89–90. [Chem Abs (1959) 53:19145e]
Glamkowski EJ (1989) (Hoechst-Roussel pharmaceuticals Inc) preparation of physostigmine analogs for treatment of memory dysfunction. US 4,914 102 (Cl. 514–232 8; A61K31/40), 03 Apr 1990, Appl 413,901, 28 Sep 1989; 6pp [Chem Abs (1990) 113:59152f]
Glamkowski EJ, Kurys BE (1989) (Hoechst-Roussel pharmaceuticals Inc) preparation of physostigmine analogs as brain acetylcholinesterase inhibitors can Pat Appl CA 2,029,265 (Cl. C07D487/04), 04 May 1991, US Appl. 431, 103 03 Nov 1989; 43pp [Chem Abs (1991) 115:232224x]
Glamkowski EJ, Chiang Y, Hamer RRL (1989a) (Hoechst-Roussel pharmaceuticals Inc) preparation of N-carbamoylphysostigmine analogs as analgesics and cholinergics. Eur Pat Appl EP 430, 201 (Cl CO7D487/04), 05 Jun 1991, US Appl 443,682, 30 Nov 1989; 18pp [Chem Abs (1991) 115: 279993n]
Glamkowski EJ, Chiang Y, Kurys BE (1989b) (Hoechst-Roussel pharmaceuticals Inc) preparation of eseroline carbonate ester derivatives as analgesic agents Eur Pat Appl EP 389,984 (Cl. CO7D487/04), 03 Oct 1990, US Appl 329,171, 27 Mar 1989; 12pp [Chem Abs (1991) 114:122343g]
Glamkowski EJ, Hamer RRL, Chiang Y, Locke KW, Huger FP, Hsu RS, Helsley GC (1989c) Abstr Pap 17th ACS-Meet , Am Chem Soc., Med. Chem. Sect, Abstr No 28, Dallas, Texas, April 9–14 [quoted as refs 55, 109, 8 and 63 in (Brossi 1990, 1992, Luo Y et al. 1990 and Takano and Ogasawara 1990, respectively)]
Goldstein A (1951) Properties and behaviour of purified plasma cholinesterase III. Competitive inhibition by prostigmine and other alkaloids with special reference to differences in kinetic behaviour. Arch Biochem Biophys 34:169–188
Goldstein A, Aronow L, Kalman SM (1974) Principles of drug action: the basis of pharmacology, vol 21, 2nd edn. Wiley, New York/London Sydney/Toronto, p 22
Goni AR (1946) Myasthenia gravis, trans Gittiner GS. Williams and Wilkins, Baltimore
Gordon JJ, Leadbeater L, Maidment MP (1978) The protection of animals against organophosphate poisoning by pretreatment with a carbamate. Toxicol Appl Pharmacol 43:207–216
Granacher RP, Baldessarini RJ (1975) Physostigmine. Its use in acute anticholinergic syndrome with antidepressant and antiparkinson drugs. Arch Gen Psych 32:375–380
Grandberg II, Ivanova TA, Yaryshev NG (1970) Indoles XII. An investigation of ring-chain tautomerism in compounds of the eserine series. Chem Het Comps 6(9):1191–1198
Greig NH (1992) Drug entry into the brain and its pharmacologic manipulation. In: handbook experimental pharmacology 103: Chapter 20, pp 487–523
Greig NH, Pei X, Soncrant TT, Ingram DK, Brossi A (1995a) Phenserine and ring C hetero-analogues: drug candidates for the treatment of Alzheimer’s disease. Med Res Rev 15(1):3–31
Greig NH, Brossi A, Soncrant TT, Holloway HW, Rapoport SI, Iijima S, Spangler EL, Ingram DK, Pei X (1995b) U.S. Pat 5,409,948 [quoted as ref 51 in (Brossi et al. 1996)]
Greig NH, Sambammurti K, Yu Q, Brossi A, Bruinsma GB, Lahiri DK (2005a) An overview of phenserine tartrate,a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res 2:281–290
Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu Q, Mamczarz J, Holloway HW, Giordano T, Chen D, Furukawa K, Sambammurti K, Brossi A, Lahiri DK (2005b) Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer ß-amyloid peptide in rodent. Proc Natl Acad Sci U S A 102(47):17213–17218
Griffith ER, Tomko MA, Tims RJ (1973) Sexual function in spinal cord-injuried patients: a review. Arch Phys Med Rehabil 54:539–543
Gustafson L, Edvinsson L, Dahlgren N, Halberg B, Risberg J, Rosén I, Fernő H (1987) Intravenous physostigmine treatment of Alzheimer’s disease evaluated by psychometric testing, regional cerebral blood flow (a CBF) measurement, and EEG. Psychopharmacol 93:31–35
Guttmann L (1949) The effect of Prostigmine on the reproductive functions in the spinal man. In: Proceedings of the 4th International Neurological Congress 2: 69, Paris Masson [quoted in (Blockmans and Steeno 1988, Chapelle et al. 1983, Guttmann and Walsh 1971)]
Guttmann L, Walsh JJ (1971) Prostigmine assessment test of fertility in spinal man. Paraplegia 9:39–51
Gysin H (1952a) (to J R Geigy A -G ) Dihydroresorcinyl carbamates. U.S. 2, 592, 890, 15 Apr 1952 [Chem Abs (1952) 46:11235e]
Gysin H (1952b ) Un nouveau group de substances à activitié insecticide. 3e Congrès International de Phytopharmacie (Paris, September) [quoted as ref 118a in (Holmstedt 1972)]
Gysin H (1954) Über einige neue insektizide. Chimia 8(9):205–210, 221; 8(10): 221–228
Gysin H, Margot A (1954) (to J R Geigy A -G ) Insecticidal phosphate esters. Ger 910, 652, May 3 1954 (Cl 12p, 10) [Chem Abs (1956) 50:10770e] [see Brit 713278 (C A 50: 1092i)]
Gysin H, Margot A (1956) (to J R Geigy A -G ) Insecticidal phosphate esters. U.S. 2, 754, 243–4 and 2,754,302, 10 July 1956 [Chem (Abs 1956) 50:12122g] [see Brit 713,278 (C A 50:1092h)]
Gysin H, Margot A, Simon C (1954a) (to J R Geigy A -G )Heterocyclic esters of dimethylcarbamic acid. U.S. 2,681,879, 22 June 1954 [Chem Abs (1955) 49:8333f] [see Swiss 281, 946 (C A 49:5532e), U.S. 2,681, 914, Swiss 281, 952 (C A 49: 5539g) U.S. 2, 681,916, Swiss 281, 947 (C A 49: 5532f)]
Gysin H, Margot A, Simon C (1954b) (to J R Geigy A -G ) 5-Pyrazolyl dimethlcarbamates. U.S. 2, 681, 915, 22 June 1954 [Chem Abs (1955) 49: 11020f]
Gysin H, Margot A, Simon C (1954c) (to J R Geigy A -G ) 2,4-Dialkyl-6-pyrimidyl dialkylcarbamates. U.S. 2,694,712, 16 Nov 1954 [Chem Abs (1955) 49:14816c]
Hamer RRL, Helsley GC, Glamkowski EJ, Chiang Y (1986) (Hoechst-Roussel pharmaceuticals Inc) preparation and testing of 1,2,3,3a,8,8a-hexahydro-3a,8-di (and 1,3a,8-tri) methylpyrrolo[2,3-b]indoles, a process for their preparation and their use as memory enhancers, antidepresants, and analgesics. Eur Pat Appl EP 253,372 (Cl. CO7D487/04), 20 Jan 1988, US Appl 885, 991, 16 July 1986; 94pp [Chem Abs (1988) 108:221937m]
Hamer RRL, Helsley GC, Glamkowski EJ, Chiang Y (1988) US Pat 4,791,107 (1988) [quoted as ref 26 in (Brossi et al. 1996)]
Hamill P (1935) Further uses of Prostigmin. Lancet March 9th 575
Hanin I, Tang XC, Kozikowski AP (1991) In: Becker R, Giacobini E (eds) Clinical and preclinical studies with huperzine, vol 1991, pp 305–313
Harel M, Quinn DM, Nair HK, Silman I, Sussman JL (1996) The X-ray structure of a transition state analog complex reveals the molecular origins of the catalytic power and substrate specificity of acetylcholinesterase. J Am Chem Soc 118:2340–2346
Haroutunian V (1995) Soc Neurosci , Abstr No 208 1 [quoted as ref 50 in (Brossi et al. 1996)]
Harris LS, Dewey WL, Howes JF, Kennedy JS, Pars H (1969) Narcotic-antagonist analgesics: interactions with cholinergic systems. J Pharmacol Exp Ther 169:17–22
Hartvig P, Wiklund L, Lindström B (1986) Pharmacokinetics of physostigmine after intravenous, intramuscular and subcutaneous administration in surgical patients. Acta Anaesthesiol Scand 30:177–182
He XS, Greig NH, Rapoport SI, Brossi A, Li Y, Yu Q (1992) Thiaphysovenine and carbamate analogues: a new class of potent inhibitors of cholinesterases. Med Chem Res 2:229–237. [see also (Flippen-Anderson et al. 1993)]
Hemsworth BA, West GB (1970) Anticholinesterase activity of some degradation products of physostigmine. J Pharm Sci 59:118–120
Hendershot LC, Forsaith J (1959) Antagonism of the frequency of phenylquinone – induced writhing in the mouse by weak analgesics and nonanalgesics. J Pharmacol Exp Ther 125:237–240
Henry TA (1924) Physostigma venenosum. In: The plant alkaloids, 2nd edn. J&A. Churchill, London, pp 415–420
Henry TA (1949) Alkaloids of Calabar bean. In: The plant alkaloids, 4th edn. J&A Churchill Ltd, London, pp 539–553. [and also earlier editions such as (Henry 1924)]
Hersh LB (1981) Inhibition of aminopeptidase and acetylcholinesterase by puromycin and puromycin analogs. J Neurochem 36(4):1594–1596
Hill RK, Barcza S (1966) Stereochemistry of the jaborandi alkaloids. Tetrahedron 22:2889–2893
Himmelsbach CK, Oberst FW, Brown RR, Williams EG (1942) Studies of the influence of Prostigmine on morphine addiction. J Pharmacol Exp Ther 76:50–56
Hino T (1961b) Synthetic approaches to calycanthaceous alkaloids. II. A synthesis of 1,1′-dimethyl-3,3′-bis(2-aminoethyl)-3,3′-bioxindole. Chem pharm bull (Tokyo) 9:979–988 (ref 5–7 cited therein)
Hino T, Ogawa K (1961) [quoted as a footnote on p 991 in (Hino 1961b) and as ref 32b in (Coxworth 1965) ˗ details have been reported in (Yamada S et al. 1963)]
Hino T, Yamada S (1963) Total synthesis of (±)-folicanthine. Tetrahedron Lett (25):1757–1760
Holmstedt B (1972) The ordeal bean of Old Calabar: The pageant of Physostigma venenosum in medicine. In: Swain T (ed) Plants in the development of modern medicine. Harvard University Press, Cambridge, 303–360 (this article is superbly illustrated)
Hunt R, Taveau Rde M (1910) On the relation between the toxicity and chemical constitution of a number of derivatives of choline and analogous compounds. J Pharmacol Exp Ther 1:303–339
Iijima S, Greig NH, Garofalo P, Spangler EL, Heller B, Brossi A, Ingram DK (1992) The long-acting cholinesterase inhibitor heptyl-physostigmine attenuates the scopolamine-induced learning impairment of rats in a 14-unit T-maze. Neurosci Lett 144:79–83
International Defence Review (1969) /Interavia/II/170–174 [quoted on p 332 of (Holmstedt 1972)]
Ireson JD (1970) A comparsion of the antinociceptive actions of cholinomimetic and morphine-like drugs. Brit J Pharmacol 40:92–101
Irvine FR (1961) Woody plants of Ghana with special reference to their uses. Oxford University Press, London, pp 402–404, 792
Irwin RL, Hein MM (1962) The inhibition of rat brian cholinesterase after administration of the dimethyl-carbamates of deoxy-demethyl-lycoramine, neostigmine or physostigmine. J Pharmacol Exp Ther 136:20–25
Irwin RL, Smith HJ (1960a) Cholinesterase inhibition by galanthamine and lycoramine. Biochem Pharmacol 3:147–148
Irwin RL, Smith HJ (1960b) The activity of galanthamine and releated compounds on muscle. Arch Int Pharmacodyn 127(3–4):314–330
Irwin RL, Smith HJ, Hein MM (1961) The activity of certain lycoramine derivatives on muscle. J Pharmacol Exp Ther 134:53–59
Isaksson K, Kissinger PT (1987) Metabolism of physostigmine in mouse liver microsomal incubations studies by liquid chromatography with dual-electrode amperometric detection. J Chromatogr 419:165–175
Iversen LL, Bentley G, Dawson G, Freedman SB, Harley EA, Iversen SD, Rupniak NMJ, Tye S, Pagella PG, Rugarli PL (1991) In: Becker R, Giacobini E (eds) Heptyl physostigmine – novel acetylcholinesterase inhibitor: biochemical and behavioral pharmacology, pp 297–304
Iwasa T, Harada S, Sato U (1981) Miticidal antibiotics C-8030 B, C, D, and physostigmine produced by Streptomyces pseudogriseolus subsp. Iriomotensis subsp. NOV. Takeda Kenkyushoho, 40(1/2):12–26 [Chem Abs (1981) 95:148651v] [see also J Takeda Res Lab 40:12 – as used and quoted as ref 9 in (Murao and Hayashi 1986)]
Jackson AH, Smith AE (1964) The protonation of tryptamine derivatives in acidic media. J Chem Soc 5516–5517 and refs therein cited [see also (Robinson B 1969)]
Jolly F (1895a) Über Myasthenia gravis pseudoparalytica. Berl klin Wschr 32:1–7 [quoted as ref 2 in (fisher 2002) and as ref 104 in (Holmstedt 1972)]
Jolly F (1895b) Pseudoparalysis myasthenica. Neurol Zbl 14:34–36 [quoted as ref 105 in (Holmstedt 1972)]
Jotkowitz S (1983) Lack of clinical efficiency of chronic oral physostigmine in Alzheimer’s disease. Ann Neurol 14:690–691
Kahane E, Lévy J (1936) Inhibition of the hydrolysis of acetylcholine by serum. CR Soc Biol 121:1596–1600 [Chem Abs (1936) 30:60169– 60171]
Kahane E, Lévy J (1937) Influence of various esterase-inhibiting agents on the pharmacodynamic activity of acetylcholine. CR Soc Biol 125:252–256 [Chem Abs (1937)31:63359 - 63361]
Karlsson E, Mbugua PM, Rodriguez-Ithurralde (1985) Anticholinesterase toxins. Pharmacol Ther 30:259–276
Kawabuchi M, Boyne AF, Deshpande SS, Cintra WM, Brossi A, Albuquerque EX (1988) Enantiomer (+) -physostigmine prevents organophosphate- induced subjunctional damage at the neuromuscular synapse by a mechanism not related to cholinesterase carbamylation. Synapse 2:139–147
Keeler JR, Hurst CG, Dunn MA (1991) Pyridostigmine use as a nerve agent pretreatment under wartime conditions. J Am Med Assoc 266(5):693–695
Kerharo J, Bouquet A (1950) Plantes médicinales et toxiques de la Cȏte d’Ivoire-Haute-Volta. Ministère de la France d’Outre-mer. Office de la Recherche Scientifique Outre-Mer, Paris, 291 pp [quoted in (Irvine 1961) and available ˗ but for consulatation only, namely not for loan as it is a rare book ˗ in the British library, London and in the library of the University of Cambridge]
Klee WA, Streaty RA (1974) Narcotic receptor sites in morphine-dependant rats. Nature 248:61–63
Klein J (2007) Phenserine. Expert Opin Investig Drugs 16(7):1087–1097
Kleinwächter (1864) Beobachtungen über die wirkung des Calabar-extrakts gegen atropin-vergiftung Berliner Klinische Wochenschrift 369–371 [quoted as ref 41 in (Holmstedt 1972), as ref 1 in (Nickalls RWD and Nickalls EA 1988) and in (Neuwinger 1996)]. “In view of the historical nature of Kleinwächter’s paper [Observations on the effect of Calabar bean extract as an antidote to atropine poisoning] it is translated fairly literally, and follows the original German closely” in (Nickalls RWD and Nickalls EA 1988)
Koelle GB (1946) Protection of cholinesterase against irreversible inactivation by di-isopropyl fluorophosphate in vitro. J Pharmacol Exp Ther 88:232–237
Koelle GB (sub-ed) (1963) Cholinesterases and anticholinesterase agents. In : Eichler O, Farah A (eds) Handbuch der experimentellen pharmakologie 15: chp 8. Springer, Berlin/Göttingen/Heidelberg
Koelle GB, Gilman A (1949) Anticholinesterase drugs. Pharmacol Rev 1:166–216
Kolosov MN, Preobrazhenskii NA (1953a) Synthetic studies in the series of indole derivatives. I. Synthesis of urethans of l-methyl-5-hydroxyindoline and 1,3-dimethyl-5-hydroxyindoline. Zh Obsh Khim 23:1563–1569 [Chem Abs (1954) 48:10729h]. J Gen Chem USSR 23:1641–1647
Kolosov MN, Preobrazhenskii NA (1953b) The synthesis of indole derivatives. II. Synthesis of methylurethans of 1,3,3-trimethyl-5-hydroxyindoline and 1,3-dimethyl-3-ethyl-5-hydroxyindoline. Zh Obsh Khim 23:1779–1784 [Chem Abs (1955) 49:295g]
Kolosov MN, Preobrazhenskii NA (1953c) Synthesis studies in the series of indole derivatives. III. Synthesis of urethans of 1,3-dimethyl-3-(ß-dimethylaminoethyl)-5-hydroxyindolin-2-one (dihydrohomoeserolinemethine). Zh Obsh Khim 23:1922–1927 [Chem Abs (1955) 49:1005c]
Kolosov MN, Metroveli LI, Preobrazhenskii NA (1953) Synthetic investigations of indole derivatives, IV. The synthesis of esermethole, homoesermethole, and homoeseroline. J Gen Chem USSR 23:2413–2149; Zh Obsh Khim 23:2027–2034 [Chem Abs (1955) 49:3208i–3210c]
Koster R (1946) Synergisms and antagonisms between physostigmine and di-isopropyl fluorophosphate in cats. J Pharmacol Exp Ther 88:39–46
Kretz E, Müller JM, Sclitter E (1952) Über die synthese eines physostigminähnlichen körpers. Helv Chim Acta 35:520–528
Kronman C, Ordentlich A, Barak D, Velan B, Shafferman A (1994) The “back door” hypothesis for product clearance in acetylcholinesterase challenged by site-directed mutagenesis. J Biol Chem 269(45):27819–27822
Kuhr RJ, Dorough HW (1976) Carbamate insecticides: chemistry, biochemistry, and toxicology, vol 18901. CRC Press Inc, Cranwood Parkway/Cleveland, p 44128
Lange W (1932) Über ester der monofluorphosphorsäure. Ber 658:1598–1601
Laqueur L (1876) Neue therapeutische indikation fuer physostigmine. Centr Med Wissensch 14:421–422
Laurent LPE (1935) Clinical observations on the use of Prostigmin in the treatment of Myasthenia gravis. Br Med J March 9:463–465
Leduc BE, Poulin O, Parisi D (1988) Au sujet d’une grossesse par insémination à partir d’un donneur quadriplégique après stimulation par la physostigmine. Can Med Assoc J 139:1071–1072
Leduc BE, Roy D, Poulin O (1992) The use of physostigmine in men with spinal cord injury with ejaculatory dysfunction. Can J Rehab 5(4):231–235
Lee TBK, Wong GSK (1991) Asymmetric alkylation of oxindoles: an approach to the total synthesis of (−)-physostigmine. J Org Chem 56:872–875. [see also (Grethe and Uskoković 1983)]
Lee J-H, Turndorf H, Poppers PJ (1975) Physostigmine reversal of antihistamine-induced excitment and depression. Anesthesiology 43:683–684
Leibholz (1892) Zwei physostigminvergiftungen. Vjschr gerichtl Med Drutte Folge 3:284–287 [quoted as ref 38 in (Holmstedt 1972)]
Levin HS, Peters BH (1984) Long-term administration of oral physostigmine and lecithin improve memory in Alzheimer’s disease. Ann Neurol 15:210
Levy-Appert-Collin M-C (1969) Thèse Doct Etat Pharm, Paris [quoted as ref 11 in (Levy-Appert-Collin 1978)]
Levy-Appert-Collin M-C (1978) Sur les propriétés anticholinestérasiques de la pseudo-akuammigine, alcaloïde du Picralima nitida Stapf (Apocynacées). Ann Pharm Franc 36(1–2):77–83 [Chem Abs (1978) 89:123231w]
Lewis JJ (1964) An introduction to pharmacology, 3rd edn. E & S Livingstone Ltd, Edinburgh/London
Lin Y, Wu X, Feng S, Jiang G, Luo J, Zho US, Vrijmoed LLP, Jones EBG, Krohn K, Steingröver K, Zrila F (2001) Five unique compounds: xyloketals from mangrove fungus Xylaria sp from the South China Sea coast. J Org Chem 66:6252–6256
Linsenmeyer TA, Perkash I (1991) Infertility in men with spinal cord injury. Arch Phys Med Rehabil 72:747–754
Lobstein JE (1935) Action of some esters, genalkaloids and glucosides on neuromuscular excitability. Cong Pharm (Liège, 1934), 107–109; Chimie & Industrie 35:890–891 [Chem Abs (1936) 30:49318–49321]
Loewi O (1921a) Über humorale übertragbarkeit der herznervenwirkung. I Mitteilung Pfluegers Arch 189:239–242
Loewi O (1921b) Über humorale übertragbarkeit der herznervenwirkung. II. Mitteilung. Pfluegers Arch 193:201–213
Loewi O (1960) An autobiographic sketch. Perspect Biol Med 4:3–25
Loewi O, Navratil E (1926a) Über humorale übertragbarkeit der herznervenwirkung. X. Mitteilung. Über das schicksal des vagusstoffs. Pfluegers Arch 214:678–688 (see, in particular, footnote 1 on p 688)
Loewi O, Navratil E (1926b) Uber humorale übertragbarkeit der herznervenwirking. XI. Mitteilung. Über den mechanismus der vaguswirkung von physostigmin und ergotamine. Pfluegers Arch 214:689–696
Long JP (1963) Structure-activity relationships of the reversible anticholinesterease agents. In: Koelle GB (sub-ed) (1963)
Long JP, Evans CJ (1967) Reversible inhibitors osf cholinesterase. In: Burger A (ed) Drugs affecting the peripheral nervous system, 1: Chapter 6, Edward Arnold/ Marcel Dekker, London/New York
Longmore RB (1966b) [(Longmore 1966a) as quoted as ref 41 in (Robinson B 1968)]
Longmore RB (1969) The absolute configurations of the alkaloids of Physostigma venenosum seeds. PhD Thesis, Victoria Manchester
Longmore RB, Robinson B (1973) Comments and further observations on the structure of eserine (physostigmine). Nat New Biol 246(155):239–240
Luo Y, Yu Q, Chrisey L, Brossi A (1990) Synthesis of (±)-physovenine and (±)-7-bromophysovenine from intermediates of the synthesis of physostigmines. Heterocycles 31(2):283–287
Luo W, Yu Q, Holloway HW, Parrish D, Greig NH, Brossi A (2005a) Synthesis of tetrahydrofurobenzofurans and dihydromethanobenzodioxepines from 5-hydro-3-methyl-3H-benzofuran-2-one. Rearrangement and ring expansion under reduction conditions on treatment with hydrides. J Org Chem 70:6171–6176
Luo W, Yu Q, Zhan M, Parrish D, Deschamps JR, Kulkarni SS, Holloway HW, Alley GM, Lahari DK, Brossi A, Greig NH (2005b) Novel anticholinesterases based on the molecular skeletons of furobenzofuran and methanobenzodioxepine. J Med Chem 48:986–994
Luo W, Yu Q, Kulkarni SS, Parrish DA, Holloway HW, Tweedie D, Shafferman A, Lahiri DK, Brossi A, Greig NH (2006) Inhibition of human acetyl˗ and butyrylcholinesterase by novel carbamates of (–)- and (+)-tetrahydrofurobenzofuran and methanobenzodioxepine. J Med Chem 49:2174–2185
Luo W, Yu Q, Holloway HW, Tweedie D, Parrish D, Brossi A, Greig NH (2007) Syntheses and anticholinesterase activities of novel 3-aminocarbonylmethylene-3-methyl-2,3-dihydrobenzofuran-5-ylcarbamates. Heterocycles 71(ii): 2413–2425
Macinnis P (2004) The killer bean of Calabar and other stories. Allan & Unwin, Crows Nest, NSW 2065, Australia
Mackay G (1909) Obituary. Douglas Argyll Robertson. Br Med J 16 Jan: 191–193 [quoted in (Holmstedt 1972)]
Main AR, Hastings FL (1966) Carbamylation and binding constants for the inhibition of acetylcholinesterase by physostigmine (eserine). Science 154:400–402
Marchbanks (1982) Biochemistry of Alzheimer’s dementia. J Neurochem 39(1):9–15
Marion L (1952) Alkaloids of Calabar bean. In: Manske RHF and Holmes HL (eds) The alkaloids, vol 2. Academic, New York, Chapter 11, pp 438–450, 494–497
Marquis JK (1983) Aluminum inhibition of human serum cholinesterase. Bull Environ Contam Toxicol 31:164–169
Marquis JK (1985) Noncholinergic mechanisms of insecticide toxicity. Trends Pharmacol Sci February: pp 59, 60
Marquis JK, Lerrick AJ (1982) Noncompetitive inhibition by aluminium, scandium and yttrium of acetylcholinesterase from Electrophorus electricus. Biochem Pharmacol 31(7):1437–1440
Marrs TC (1993) Organophosphate poisoning. Pharmacol Ther 58:51–66
Marta M, Castellano C, Oliverio A, Pavone F, Pagella PG, Brufani M, Pomponi M (1988) New analogs of physostigmine: alternative drugs for Alzheimer’s disease? Life Sci 43(23):1921–1928
Marx JL (1987) Alzheimer’s drug trial put on hold. Science 238:1041–1042
Matsumura F (1975) Toxicology of insecticides. Chapter 3.4 (carbamate insecticides). Plenum press, New York/London. pp 78–85
McCombie H, Saunders BC (1946) Alkyl fluorophosphonates: preparation and physiological properties. Nature 157:287–289
Merck Index (1989) 11th edn, Index no 7357 [as quoted as ref 3 in (Yu et al. 1991) – nowhere in this Index is “urinary retention” even cited]
Merck Index (2001) 13th edn, Index no 1024(a), 3733(b), 5527(c), 6755(d) 6898(e), 7064(f), 7468(g), 7469(h), 7470(i), 7505(j), 8627 (k), 8882(l), 8893(m), 8909(n)
Metcalf RL (1955) Organic insecticides. Their chemistry and mode of action. Chapter 12 (carbamates). Interscience Publishers Inc, New York/London, pp 317–329
Miller D (2007) A solution for the heart. The life of Sydney ringer (1836–1910) professor of medicine, University College London benefactor of St Mary’s Lastingham, North Yorkshire. The Physiological Society (available at St Mary’s Church, Lastingham, North Yorkshire)
Mohs RC, Davis BM, Johns CA, Mathé AA, Greenwald BS, Horvath TB, Davis KL (1985) Oral physostigmine treatment of patients with Alzheimer’s disease. Am J Psychiatry 142:28–33
Moroi SE, Lichter PR (1996) Ocular pharmacology. In: [Gilman et al. (eds) 1996], Chapter 65, pp 1619–1645
Muhtadi FJ, El-Hawary SS (1989) Analytical profile of physostigmine salicylate. In: Florey K (ed) Analytical profiles of drug substances, vol 18. Academic, San Diego/New York/Berkeley/Boston/London/Sydney/Tokyo/Toronto, pp 289–350
Muñoz-Ruiz P, Rubio L, Garcia-Palomero E, Dorronsoro I, del Monte-Millán M, Valenzuela R, Usán P, de Austria C, Bartolini M, Andrisano V, Bidon-Chanal A, Orozco M, Luque FJ, Medina M, Martinez A (2005) Design, synthesis and biological evaluation of dual binding site acetylcholinesterase inhibitors: new disease-modifying agents for Alzheimer’s disease. J Med Chem 48:7223–7233. and refs quoted therein
Murao S, Hayashi H (1986) Physostigmine and N8-norphysostigmine, insecticidal compounds from Streptomyces sp. Agric Biol Chem 50:523–524
Nachmansohn D, Lederer (1939) Sur la biochimie de la cholinestérase. I. – Préparation de l’enzyme, rôle des groupements – SH. Bull Soc Chim Biol 21:797–808
Nachmansohn D, Rothenberg MA (1945) Studies on cholinesterase. I. On the specificity of the enzyme in nerve tissue. J Biol Chem 158:653–666
Nachmansohn D, Wilson IB (1951) The enzymic hydrolysis and synthesis of acetylcholine. Adv Enzymol 12:259–339
Neuwinger HD (1996) Physostigma Venenosum Balfour. In: African ethnobotany poisons and drugs chemistry, pharmacology, toxicology, translated by Porter A and the author from the original German edition Afrikanische arzneipflanzen und jagdgifte. Chapman & Hall, London/Glasgow/Weinheim/New York/Tokyo/Melbourne/Madras, XVI, 699–707, colour plate -VI (this publication also includes a useful chapter upon literature concerning traditional medicine in Africa)
Nickalls RWD, Nickalls EA (1988) The first use of physostigmine in the treatment of atropine poisoning. Anesth 43:776–777
Nogrady T (1985) Medicinal chemistry, a biochemical approach. Oxford University Press, Inc, New York, Oxford, pp 24(a), 120(b), 266–270(c), 304–305(d), 306(e), 308(f)
Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, Sussman JL, Verschueren KHG, Goldman A (1992) The α/ß hydrolase fold. Protein Eng 5(3):197–211
Orzechowski G, Hundreiser M (1936) Comparative studies of genalkaloids and the corresponding alkaloids. Klin Wochsohr 15:481–483 [Chem Abs (1936) 30:68237)
Pardridge WM (1988) Recent advances in blood-brain barrier transport. Ann Rev Pharmacol Toxicol 28:25–39
Pasteur L (1858) Chimie organique – mémoire sur la fermentation de l’acide tartrique. CR Seances. Acad Sci 46:615–618
Pei X, Greig NH, Brossi A (1998) Synthesis and biological evaluation of (±)-3a-phenyl congeners of physostigmine and phenserine. Heterocycles 49:437–444
Pei X, Yu Q, Holloway HW, Brossi A, Greig NH (1999) Synthesis and biological evaluation of ring-C opened analogues of the cholinesterase inhibitors physostigmine, phenserine and cymserine. Med Chem Res 9:50–60
Perola E, Cellai L, Lamba D, Filocamo L, Brufani M (1997) Long chain analogues of physostigmine as potential drugs for Alzheimer’s disease: new insights into the mechanism of action in the inhibition of acetylcholinesterase. Biochim Biophys Acta 1343:41–50
Petcher TJ, Pauling P (1973) Cholinesterase inhibitors: structure of eserine. Nature 241:277
Peters BH, Levin HS (1979) Effects of physostigmine and lecithin on memory in Alzheimer’s disease. Ann Neurol 6:219–221
Pleuvry BJ, Tobias MA (1971) Comparison of the antinociceptive activities of physostigmine, oxotremorine and morphine in the mouse. Brit J Pharmacol 43:706–714
Polonovski M (1916) No 8 – Étude sur les alcaloides de la fève de Calabar (V). Action de l’isocyanate de phényle: homologues phényliques de l’ésérine et de la génésérine. Bull Soc Chim France 4th ser 19:46–59
Polonovski M, Combemale P (1923) Action de la génésérine sur les secretions salivaire et pancréatique. CR Soc Biol de Lille, 88:881–883 [Chem Abs (1923) 17:2326] and [quoted in (Henry 1924)]
Polonovski M, Nitzberg C (1915a) No 30. ˗ Etude sur les alcaloïdes de la fève. de Calabar (II). La génésérine, nouval alcaloïde de la fève. Bull Soc Chim France 4th ser 17:244–256
Polonovski M, Pelou A, Schmitt H (1952) Potentiation of the vascular effects of two nicotinic phenoxyisopropylamines by anticholinesterasic compound. CR Soc Biol 146:1873–1874 [Chem Abs (1953) 47:11517b]
Pomponi M (1989) Comment De bello physostigminico I. FEBS 252(1,2):158
Pomponi M, Giacobini E, Brufani M (1990) Present state and future development of the therapy of Alzheimer disease. Aging 2(2):125–153
Pomponi M, Giandina B, Gatta F, Marta M (1992) Physostigmine and tetrahydroaminoacridine analogs as alternative drugs for the treatment of Alzheimer’s disease. Med Chem Res 2:306–327
Poobrasert O, Chal H, Pezzuto JM, Cordell GA (1996) Cytotoxic degradation product of physostigmine. J Nat Prod 59:1087–1089
Poobrasert O, Cordell GA, Bobzin SC (1997) Blue degradation products of rubreserine. J Nat Prod 60:578–580
Porter GR, Rydon HN, Schofield JA (1958) Nature of the reactive serine residue in enzymes inhibited by organo-phosphorus compounds. Nature 182:927
Pratviel G, Bernadou J, Meunier B, Paoletti C (1985) Regioselective 2′-0 sugar arylation in adenine nucleosides by the elliptinium acetate, an anti tumor agent. Nucleosides Nucleotides Nucleic Acids 4:143–146
Quinn DM (1987) Acetylcholinesterase: enzyme structure, reaction dynamic, and virtual transition states. Chem Rev 87:955–979
Rawicki H, Lording DW (1988) Assisted fertility in complete paraplegia: case report. Paraplegia 26:401–404
Rawicki HB, Hill S (1991) Semen retrieval in spinal cord injured man. Paraplegia 29:443–446
Raymond-Hamet, Vincent D, Parant M (1956) Action of some sympathicosthenic alkaloids on cholinesterases. CR Soc Biol 150:1384–1386 [Chem Abs (1957) 51:5989g]
Rees JMH, Cox B, Tanzil S, Newboult L, Kimber K, Robinson B (1989) In vitro opioid activity of 9,10-dimethyl-6-hydroxyechiboline, and its 10-allyl derivative. Adv Biosci (Oxford) 75 (Prog Opioid Res): 93–96 [Chem Abs (1989), 111:208991s] In: Cros J, Meunier J-Cl and Hamon M (eds) Proceedings of the 19th international narcotics research conference, held at Albi, France on 3rd-8th July 1988, Pergamon Press, Oxford, New York, Beijing, Frankfurt, São Paulo, Sydney, Tokyo, Toronto
Remen L (1932) Zur pathogenese und therapie der Myasthenia gravis pseudoparalytica. Dtsch Z Nervenheilk 128: 66–78 [quoted in (Neuwinger 1996) and as ref 5 in (Viets 1965)]
Reynalds GF (1899) Two cases of poisoning I by Calabar bean. J Trop Med 1(8):206–207
Ringer S, Murrell W (1877) On the use of physostigma in some nervous affections. Lancet 912–914, 950–952
Ripoll DR, Faerman CH, Axelson PH, Silman I, Sussman JL (1993) An electrostatic mechanism for substrate guidance down the aromatic gorge of acetylcholinesterase. Proc Natl Acad Sci U S A 90:5128–5132
Robertson DA (1863) The Calabar bean as a new agent in ophthalmic medicine. Edinb Med J 8:193–204, pt2, 815–820
Robinson R (1917b) LXXV. A theory of the mechanism of the phytochemical synthesis of certain alkaloids. J Chem Soc 111:876–899
Robinson R (1925) Suggestion from [quoted in (Stedman and Barger 1925)]
Robinson B (1964a) Alkaloids of Physostigma venenosum. Part I. The structure of physovenine. J Chem Soc:1503–1506
Robinson B (1964b) Alkaloids of Physostigma venenosum seeds. West Afr Pharm 6(5 and 6):92–96, 110–112
Robinson B (1965d) Unpublished results [quoted as ref 46 in (Robinson B 1968)]
Robinson B (1968) Alkaloids of the Calabar bean, In: Manske RHF (ed) The alkaloids chemistry and physiology, vol 10. Academic, New York/London, chapter 5, pp 383400
Robinson B (1971) Alkaloids of the Calabar bean. In: Manske RHF (ed) The alkaloids chemistry and physiology, vol 13. Academic, New York/London, Chapter 4, pp 213–226
Robinson B (1988a) The Calabar bean and its alkaloids: from magic to medicine. West African J Pharmacol Drug Res (J Quest – Africian de pharmacologie et de recherche sur les medicaments) 8:1–13 – a synopsis of presentations by the author to the 17th annual scientific conference concerning alkaloids in medicine of the West African Society for Pharmacology (Societe Quest-Africane de Pharmacologie), held at the University of Calabar, Nigeria, April 10th–13th, 1988
Robinson T (1988b) Alkaloids in bio-organic chemistry. Freeman, San Francisco and London, Chapter 18, p 168
Robinson B (2002) Syntheses of (-)-physostigmine, with particular emphasis upon the clarification of two enigmatic early synthetic approaches. Heterocycles, 57:1327–1352 [see also (Ault 2008)]
Robinson B (2012) God’s hydrogen bond and its role in the life process. To be published
Robinson B, Robinson JB (1968) The anti-acetylcholinesterase activities of the alkaloids of Physostigma venenosum seeds. J Pharm Pharmacol 20(S1):213S–217S
Robinson B, Rees JMH, Cox B (1987) (Victoria University of Manchester) new organic compounds having opioid properties. Int Pat Appl PCT/GB87/00457 30 June 1987 – see also Eur Pats Bull, Pub no EP0312539(A1)-1989-04-26
Robinson B, Rees JMH, Cox B (1988) (Victoria University of Manchester) Preparation of new echiboline derivatives having opioid properties. PCT Int. Appl. WO 88, 00,193 (Cl. CO7D487/08), 14 Jan 1988, GB appl. 86/16,089, 02 Jul 1986; 40pp. [Chem Abs (1988) 109:73726v]
Roddick JG (1989) The acetylcholinesterase inhibitory activity of steroidal glycoalkaloids and their aglycones. Phytochemistry 28(10):2631–2634
Rodin FH (1947) Eserine: its history in the practice of ophthalmology. Am J Ophthal 30:19–28
Rosenberry TL (1975) Acetylcholinesterase. Adv Enzymol 43:103–218
Rothenburg MA, Nachmansohn D (1947) Studies on cholinesterase III. Purification of the enzyme from electric tissue by fractional ammonium sulfate precipitation. J Biol Chem 168:223–231
Rydon HN (1958) A possible mechanism of action of esterases inhibitable by organo-phosphorus compounds. Nature 182:928–929
Salway AH (1911) CCXLII. Chemical examination of Calabar beans. J Chem Soc 99: 2148–2159 {see also Pharm J Pharmacist, 87: 719; Proc Chem Soc 27:273–274 [Chem Abs (1912) 6: 792]}
Sano M, Bell K, Marder K, Stricks L, Stern Y, Mayeux R (1993) Safety and efficacy of oral physostigmine in the treatment of Alzheimer’s disease. Clin Neuropharmacol 16(1):61–69
Santesson CG (1926) Einige drogen aus dem Kamerun-Gebiete und ihre einheimische verwendung. Archiv für Botanik, 20,8:1–34 [quoted in (Dalziel 1948 and Irvine 1961)] [see also (Dalziel 1937) as quoted in Oliver-Bever BEP (1986) Medicinal plants in tropical West Africa. Cambridge University Press, Cambridge, London, New York, New Rochelle, Melbourne, Sydney, 41 274]
Saunders BC (1947) Toxic fluorophosphonates and related compounds. In: A paper delivered at an ordinary meeting of the chemical society, January 9th (reported in Chem Ind 117)
Schneck HJ, Tempel G, Rupreht J (1989) Zur pharmacologie des physostigmin. Drug Dev Eval I5:1–9 [Chem Abs (1990) 112:111378e]
Schoene K, Steinhanses J, Oldiges H (1976) Protective activity of pyridinium salts against Soman poisoning in vivo and in vitro. Biochem Pharmacol 25:1955–1958
Schönenberger B, Jacobson AE, Brossi A, Streaty R, Klee WR, Flippen-Anderson JL, Gilardi R (1986b) Comparison of (-)-eseroline with (+)-eseroline and dihydroseco analogues in antinociceptive assays: confirmation of rubreserine structure by X-ray analysis. J Med Chem 29:2268–2273
Shaw KTY, Utsuki T, Rogers J, Yu Q, Sambamurti K, Brossi A, Ge YW, Lahiri DK, Greig NH (2001) Phenserine regulates translation of ß-amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development. Proc Natl Acad Sci U S A 98(13):7605–7610
Silman I, Sussman JL (2000) Structural studies on acetylcholinesterase. In: Giacobini E (ed) 2000
Silver A (1974a) Anticholinesterases. In: Silver A (1974b) Chapter 11, pp 449–488
Silver A (1974b) The biology of cholinesterases. In: Newberger A and Tatum EL (eds) North-Holland research monographs. Frontiers of biology. North-Holland publishing company, Amsterdam, Oxford 36: chapters 1–11, pp 1–596
Slaughter D, Parsons JC, Munal HD (1940) New clinical aspects of the analgesic action of morphine. J Am Med Assoc 115:2058–2060
Slaughter D, Treadwell CR, Gales JW (1943) Some new aspects of morphine action: influence of prostigmine methlsulfate on excretion. J Lab Clin Med 28:1199–1203
Solter AW (1965) Cholinergic anionic receptors II. Examination of a conformationally restricted analog of acetylcholine. J Pharm Sci 54:1755–1757
Soreq H, Zakut H (1993) Human cholinesterases and anticholinesterases. Academic, Inc. Harcourt Brace & Company, San Diego/New York/Boston/London/Sydney/Tokyo/Toronto, pp 1–301
Soreq H, Gnatt A, Loewenstein Y, Neville LF (1992) Excavations into the active-site gorge of cholinesterases. Trends Biochem Sci 17 Sept:353–358
Sourkes TL (1966) Nobel prize winners in medicine and physiology 1901–1965. Abelard-Schuman, London/New York/Toronto, pp 191–200(a), 278–290(b), 407–420(c)
Stedman E (1926) Studies on the relationships between chemical constitution and physiological action. Part 1. Position isomerism in relation to the miotic activity of some synthetic urethanes. Biochem J 20:719–734
Stedman (1929) Studies on the relationship between chemical constitution and physiological action. Part II. The miotic activity of urethanes derived from the isomeric hydroxybenzyldimethylamines. Biochem J 23:17–24
Stedman E, Barger G (1925) XLII. Physostigmine (eserine). Part III. J Chem Soc 127:247–258
Stedman E, Stedman E (1929) The methylurethanes of the isomeric α-hydroxyphenylethyldimethylamines and their miotic activity. J Chem Soc 0:609–617
Stedman E, Stedman E (1931a) The methylurethanes of α-3-hydroxy-4-methoxyphenylethyldimethylamine and α-4-hydroxy-3-methoxyphenylethyldimethylamine and their mioic activities. J Chem Soc:1125–1131
Stedman E, Stedman E (1931b) Studies on the relationship between chemical constitution and physiological action III. The inhibitory action of certain synthetic urethanes on the activity of liver esterate. Biochem J 25:1147–1167
Stedman E, Stedman E (1932) Studies on the relationship between chemical constitution and physiological action IV. The inhibitory action of certain synthetic urethanes on the activity of esterases. Biochem J 26:1214–1222
Stedman E, Stedman E, Easson LH (1932) Choline-esterase, an enzyme present in the blood-serum of the horse. Biochem J 26:2056–2066
Stedman E, Stedman E, White AC (1933) A comparison of the choline-esterase activities of the blood-sera from various species. Biochem J 27:1055–1060
Stempel A, Aeschlimann JA (1956) Synthetic analogs of physostigmine. In: Blicke FF, Cox RH (eds) Medicinal Chemistry, Chapter 4. Chapman & Hall, London
Stenberg VI, Narain NK, Singh SP, Obenauf RH, Albright MJ (1977) Carbon-13 and nitrogen-15 nuclear magnetic resonace of physostigmine (eserine). J Heter Chem 14:407–410
Stern Y, Sano M, Mayeux R (1987) Effects of oral physostigmine in Alzheimer’s disease. Ann Neurol 22:306–310
Stevens JR, Beutel RH (1941) Physostigmine substitutes. J Am Chem Soc 63:308–311
Sugasawa S, Murayama M (1958b) Synthesis of homoesermethole. Chem Pharm Bull (Tokyo) 6:200–203
Sussman JL, Silman I (1992) Acetylcholinesterase: structure and use as a model for specific cation-protein interactions. Curr Opin Struct Biol 2:721–729
Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I (1991) Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science 253:872–879
Svoboda GH (1963) Alkaloids. History, preparation and use. In: Parolla EA et al. (eds) 1963, pp 778–809
Takano S, Ogasawara K (1989) Alkaloids of the Calabar bean. In: Brossi (ed) The alkaloids chemistry and pharmacology, vol 36. Academic, San Diego/New York/Berkeley/Boston/London/Sydney/Tokyo/Toronto. Chapter 5, pp 225–251
Tan RC, Truong TN, McCammon JA, Sussman JL (1993) Acetylcholinesterase: electrostatic steering increases the rate of ligand binding. Biochemistry 32:401–403
Tanaka T, Weitzell R, Starke K (1978) High selectivity of rauwolscine for presynaptic α-adrenoceptors. Eur J Pharmacol 52:239–240
Tarabulcy E (1972) Sexual function in the normal and in paraplegia. Paraplegia 10:201–208
Taylor WI (1963) Alkaloids survey. In: Parolla EA et al. (eds) 1963, pp 758–778
Taylor P (1996) Anticholinesterase agents. In: Gilman et al. (eds) 1996, Chapter 8, pp 161–176
Taylor P, Radić Z (1994) The cholinesterases: from genes to proteins. Annu Rev Pharmacol Toxicol 34:281–320
Thal LJ (1991) Physostigmine in Alzheimer’s disease. In: Becker R and Giacobini E (eds) 1991, pp 209–215
Thal LJ, Fuld PA (1983) Memory enhancement with oral physostigmine in Alzheimer’s disease. N Engl J Med 308(12):720–721
Thal LJ, Fuld PA, Masur DM, Sharpless NS (1983) Oral physostigmine and lecithin improve memory in Alzheimer’s disease. Ann Neurol 13:491–496
Thomsen T, Kewitz H, Bickel U, Straschill M, Holl G (1991) Preclinical and clinical studies with galanthamine, In: Becker R and Giacobini E (eds) 1991, pp 329–336
Triggle DJ, Mitchell JM, Filler R (1998) The pharmocolgy of physostigmine. CNS Drug Rev 4(2):87–136. and refs therein cited
Tuovinen K, Kaliste-Korhonen E, Raushel FM, Hänninen O (1999) Success of pyridostigmine, physostigmine, eptastigmine and phosphotriesterase treatments in acute sarin intoxication. Toxicology 134:169–178
Utsuki T, Yu QS, Davidson D, Chen D, Holloway HW, Brossi A, Sambamurti K, Lahiri DK, Greig NH, Giordano T (2006) Identification of novel small molecule inhibitors of amyloid precursor synthesis as a route to lower Alzheimer’s disease amyloid-ß peptide. J Pharmacol Exp Ther 318(2):855–862
van Rossum JM, Cornelissen MJWJ, de Groot CTP, Hurkmans JATM (1960) A new view on an old drug: pilocarpine. Experientia 8:373–375
Viets H (1965) The miracle at St Alfege’s. Med Hist 9:184–186
Viets HR, Schwab RS (1935) Prostigmin in the diagnosis of Myasthenia gravis. N Engl J Med 213:1280–1283 [quoted in (Viets 1965)]
Vincent D (1938) L’acétylcholine et son rôle dans l’organisme animal. Thèse Doctorat ès sciences, Lyon [quoted as ref 11 in (Vincent and Beaujard 1943 – see also other refs quoted in this paper)]
Vincent D, Beaujard P (1943) Recherches sur les alcaloïdes inhibiteurs de la cholinesterase. Bull Soc Chim Biol (travaux des membres) 25:1358–1364
Vincent D, Beaujard P (1945) La détection des alcaloïdes inhibiteurs de la cholinestérase. (esérine en particulier). Applications toxicologiques. Ann Pharm Franc 3:22–26 [Chem Abs (1946) 40:52045)
Vincent D, Maugein A (1942b) Biochemical determination of eserine by its anticholinesterase action. Its use in materia medica and pharmacy. Bull Sci Pharmacol 49:165–167 [Chem Abs (1944) 38:55195]
Vincent D, Segonzac G, Marques-Vincent MC (1961) Action of some anticholinesterases on the monoamine oxidase of the brain. CR Soc Biol 155:2446–2448 [Chem Abs (1962) 57:5253h]
von Graefe A (1863) Ueber Calabar bohne. Deutsche Klin 15:285–286; Arch Ophthalmol 9:87–128
Walker MB (1934) Treatment of Myasthenia gravis with physostigmine. Lancet 223:1200–1201
Walker MB (1935) Case showing effect of Prostigmine on Myasthenia gravis. Proc R Soc Med 28:759–761
Weber A (1876) Ueber Calabar und seine therapeutische verwendung. Graefe Arch Klin Exp Ophthal 22:215–275[quoted as ref 22 in (Anon 1970)]
Weber A (1877) Die ursache des glaucoma. Graefe Arch Klin Exp Ophthal 23:1–91 [quoted as ref 23 in (Anon 1970)]
Whitehouse PJ, Price DL, Struble RG (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239
Wildman, WC (1960) Alkaloids of the amaryllidacesae IV. Alkaloids derived from dibenzofuran. In: Manske RHF (ed) The alkaloids chemistry and physiology. Academic, New York/London, 6 (supplement to Volumes I and II): Chapter 9, pp 338–343, 410–412
Willis T (1672) De anima brutorum. Oxford, England: Theatro Sheldoniano, 404–406 [quoted as ref 3 in (Drachman 1994)]
Wilson IB (1960) Acetylcholinesterase. In: Bayer PD, Lardy H and Myrbäck (eds) The Enzymes, vol 4. 2nd edn. Academic, New York/London, Chapter 30, pp 501–520
Wilson IB (1963) Inhibition of acetylcholinesterase. In: Hochster RM and Quastel JH (eds) 1963, Chapter 24, pp 193–204
Wilson IB, Bergmann F (1950) Studies on cholinesterase VII. The active surface of acetylcholine esterase derived from effects of pH on inhibitors. J Biol Chem 185:479–489
Wilson IB, Hatch MA, Ginsburg S (1960) Carbamylation of acetylcholinesterase. J Biol Chem 235:2312–2315
Wilson IB, Harrison MA, Ginsburg S (1961) Carbamyl derivatives of acetylcholinesterase. J Biol Chem 236:1498–1500
Witkop B (1998) From the ordeal bean (physostigma venenosum) to the ordeal of Alzheimer’s disease - some of the legacy of Percy Lavon Julian (1899–1975). Heterocycles 49:9–27
Wright CI (1941) The inactivation of choline esterase by morphine and derivatives. J Pharmacol Exp Ther 72:45–46
Wright SP (1976) Usefulness of physostigmine in imipramine poisoning. A dramatic response in a child resistant to other therapy. Clin Pediatr 15(12):1123–1128
Wright JLC (1984) A new antibiotic from the marine bryozoans flustra foliaceae. J Nat Prod 47(5):893–895
Yamada S, Hino T, Ogawa K (1963) The reductive cyclisation of 1-methyl-3-aminoalkyloxindoles with lithium aluminium hydride. Chem Pharm Bull (Tokyo) 11:674–678
Yarkony GM (1990) Enhancement of sexual function and fertility in spinal cord-injured males. Am J Phys Med Rehabil 69:81–87
Yu Q, Atack JR, Rapoport SI, Brossi A (1988a) Synthesis and anticholinesterase activity of (-)-N1-norphysostigmine, (-)-eseramine, and other N(1)-substituted analogues of (-)-physostigmine. J Med Chem 31:2297–2300
Yu Q, Atack JR, Rapoport SI, Brossi A (1988b) Carbamate analogues of (−)-physostigmine: in vitro inhibition of acetyl- and butyrylcholinesterase. FEBS Lett 234(1):127–130
Yu Q, Yeh HJC, Brossi A, Flippen-Anderson JL (1989) Geneserine and geneseroline revisited: acid-base catalyzed equilibria of hexahydropyrrolo-[2,3-b]-indole N-oxides with hexahydro-1,2-oxazino-[5,6-b]-indoles. J Nat Prod 52:332–336
Yu Q, Liu C, Brzostowaka M, Chrisey L, Brossi A, Greig NH, Atack JR, Soncrant TT, Rapoport SI, Radunz H-E (1991) Physovenines: efficient synthesis of (−)- and (+)-physovenine and synthesis of carbamate analogues of (−)-physovenine. Anticholinesterase activity and analgesic properties of optically active physovenines. Helv Chim Acta 74:761–766
Yu Q, Greig NH, Holloway HW, Brossi A (1999) 4′-Hydroxyphenylcarbamates of (3a S)-eseroline and (3a S)-N(1)noreseroline: potential metabolites of the Alzheimer’s anticholinesterase drug phenserine. Heterocycles 50:95–102
Yu Q, Luo W, Holloway HW, Utsuki T, Perry TA, Lahiri DK, Greig NH, Brossi A (2003) Racemic N1-norphenserine and its enantiomers: unpredicted inhibition of human acetyl- and butyrylcholinesterase and β-amyloid precursor protein in vitro. Heterocycles 61:529–539
Yu Q, Holloway HW, Luo W, Lahiri DK, Brossi A, Greig NH (2010) Long-acting anticholinesterases for Myasthenia gravis: synthesis and activities of quaternary phenylcarbamates of neostigmine, pyridostigmine and physostigmine. Biorg Med Chem 18:4687–4693
Zhao B, Moochhala SM, Tham S (2004) Biologically active components of Physostigma venenosum. J Chrom B 812:183–192
Zhu X-D, Cuadra G, Brufani M, Maggi T, Pagella PG, Williams E, Giacobini E (1996) Effects of MF-268, a new cholinesterase inhibitor, on acetylcholine and biogenic amines in rat cortex. J Neurosci Res 43:120–126
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature B.V.
About this chapter
Cite this chapter
Robinson, B. (2023). Biological Activities of the Alkaloids of the Calabar Bean. In: The Calabar Bean and its Alkaloids. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1191-1_10
Download citation
DOI: https://doi.org/10.1007/978-94-024-1191-1_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1190-4
Online ISBN: 978-94-024-1191-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)