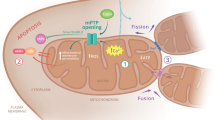
Abstract
Mitochondria play critical roles in neuronal function and emerging evidence shows that mitochondrial dysfunction is essential in the pathogenesis of several major neurological diseases, including neurodegenerative disorders and cerebral ischemia. The present chapter centers on the roles of the functionally diverse mitochondrial protein, mortalin. Since its initial discovery in mouse embryonic fibroblasts and identification of its association with cellular mortality, subsequent research efforts have recognized mortalin as having potential roles in the maintenance of mitochondrial homeostasis, energy generation, mitochondrial import of nuclear-encoded proteins, and chaperoning of misfolded proteins. Dysfunction of mortalin has been implicated in a variety of neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and brain tumors in addition to brain ischemia. This chapter will present the associative evidence implicating the protein as being involved in neurological diseases, as well as attempt to synthesize potential mechanisms by which mortalin participates in the processes of these conditions.
Jinghua Jin and Travis J. Cook have contributed to the manuscript equally.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Adam AC, Bornhövd C, Prokisch H, Neupert W, Hell K (2006) The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J 25:174–183
Alves da Costa C, Checler F (2011) Apoptosis in Parkinson’s disease: Is p53 the missing link between genetic and sporadic Parkinsonism? Cell Signal 23:963–968
Alves da Costa C, Paitel E, Vincent B, Checler F (2002) Alpha-synuclein lowers p53-dependent apoptotic response of neuronal cells. Abolishment by 6-hydroxydopamine and implication for Parkinson’s disease. J Biol Chem 277:50980–50984
Alves da Costa C, Sunyach C, Pardossi-Piquard R, Sévalle J, Vincent B, Boyer N, Kawarai T, Girardot N, St George-Hyslop P, Checler F (2006) Presenilin-dependent gamma-secretase-mediated control of p53-associated cell death in Alzheimer’s disease. J Neurosci 26:6377–6385
Association AS (2010) 2010 Alzheimer’s disease facts and figures. Alzheimers Dement 6:158–194
Blum D, Wu Y, Nissou MF, Arnaud S, Alim-Louis-Benabid, Verna JM (1997) p53 and Bax activation in 6-hydroxydopamine-induced apoptosis in PC12 cells. Brain Res 751:139–142
Braak H, Del Tredici K, Rüb U, De Vos R, Jansen Steur E, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Bretaud S, Allen C, Ingham PW, Bandmann O (2007) p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson’s disease. J Neurochem 100:1626–1635
Burbulla LF, Schelling C, Kato H, Rapaport D, Woitalla D, Schiesling C, Schulte C, Sharma M, Illig T, Bauer P, Jung S, Nordheim A, Schöls L, Riess O, Krüger R (2010) Dissecting the role of the mitochondrial chaperone mortalin in Parkinson’s disease: functional impact of disease-related variants on mitochondrial homeostasis. Hum Mol Genet 19:4437–4452
Calvo SE, Mootha VK (2010) The mitochondrial proteome and human disease. Annu Rev Genomics Hum Genet 11:25–44
Cardoso S, Moreira P, Agostinho P, Pereira C, Oliveira C (2005) Neurodegenerative pathways in Parkinson’s disease: therapeutic strategies. Curr Drug Targets CNS Neurol Disord 4:405–419
Castellani RJ, Rolston RK, Smith MA (2010) Alzheimer disease. Dis Mon 56:484–546
Cenini G, Sultana R, Memo M, Butterfield DA (2008) Elevated levels of pro-apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer’s disease. J Cell Mol Med 12:987–994
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14:1505–1517
Choi J, Forster MJ, Mcdonald SR, Weintraub ST, Carroll CA, Gracy RW (2004) Proteomic identification of specific oxidized proteins in ApoE-knockout mice: relevance to Alzheimer’s disease. Free Radic Biol Med 36:1155–1162
Crowe RR, Vieland V (1999) Report of the Chromosome 5 Workshop of the Sixth World Congress on Psychiatric Genetics. Am J Med Genet 88:229–232
da Costa CA, Sunyach C, Giaime E, West A, Corti O, Brice A, Safe S, Abou-Sleiman PM, Wood NW, Takahashi H, Goldberg MS, Shen J, Checler F (2009) Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson’s disease. Nat Cell Biol 11:1370–1375
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
De Mena L, Coto E, Sánchez-Ferrero E, Ribacoba R, Guisasola LM, Salvador C, Blázquez M, Alvarez V (2009) Mutational screening of the mortalin gene (HSPA9) in Parkinson’s disease. J Neural Transm 116:1289–1293
Deocaris CC, Kaul SC, Wadhwa R (2008) From proliferative to neurological role of an hsp70 stress chaperone, mortalin. Biogerontology 9:391–403
Deocaris CC, Kaul SC, Wadhwa R (2009) The versatile stress protein mortalin as a chaperone therapeutic agent. Protein Pept Lett 16:517–529
Dick FD, De Palma G, Ahmadi A, Osborne A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A, Group GS (2007) Gene-environment interactions in parkinsonism and Parkinson’s disease: the Geoparkinson study. Occup Environ Med 64:673–680
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55: 259–272.
Gabriele JP, Chong VZ, Pontoriero GF, Mishra RK (2005) Decreased expression of a 40-kDa catecholamine-regulated protein in the ventral striatum of schizophrenic brain specimens from the Stanley Foundation Neuropathology Consortium. Schizophr Res 74:111–119
Gabriele JP, Pontoriero GF, Thomas N, Ferro MA, Mahadevan G, Maccrimmon DJ, Pristupa ZB, Mishra RK (2010a) Antipsychotic drug use is correlated with CRP40/mortalin mRNA expression in the dorsolateral prefrontal cortex of human postmortem brain specimens. Schizophr Res 119:228–231
Gabriele N, Pontoriero GF, Thomas N, Shethwala SK, Pristupa ZB, Gabriele JP (2010b) Knockdown of mortalin within the medial prefrontal cortex impairs normal sensorimotor gating. Synapse 64:808–813
Gloeckner CJ, Schumacher A, Boldt K, Ueffing M (2009) The Parkinson disease-associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. J Neurochem 109:959–968
Harding AE (1981) Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 104:589–620
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Hartmann A, Michel PP, Troadec JD, Mouatt-Prigent A, Faucheux BA, Ruberg M, Agid Y, Hirsch EC (2001) Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson’s disease? J Neurochem 76:1785–1793
Hong KS, Mcinnes LA, Service SK, Song T, Lucas J, Silva S, Fournier E, León P, Molina J, Reus VI, Sandkuijl LA, Freimer NB (2004) Genetic mapping using haplotype and model-free linkage analysis supports previous evidence for a locus predisposing to severe bipolar disorder at 5q31–33. Am J Med Genet B Neuropsychiatr Genet 125B:83–86
Hooper C, Meimaridou E, Tavassoli M, Melino G, Lovestone S, Killick R (2007) p53 is upregulated in Alzheimer’s disease and induces tau phosphorylation in HEK293a cells. Neurosci Lett 418:34–37
Jallon P, Latour P (2005) Epidemiology of idiopathic generalized epilepsies. Epilepsia 46(Suppl 9):10–14
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J (2006) Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol Cell Proteomics 5:1193–1204
Jin J, Davis J, Zhu D, Kashima DT, Leroueil M, Pan C, Montine KS, Zhang J (2007a) Identification of novel proteins affected by rotenone in mitochondria of dopaminergic cells. BMC Neurosci 8:67
Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J (2007b) Identification of novel proteins associated with both alpha-synuclein and DJ-1. Mol Cell Proteomics 6:845–859
Kanai M, Ma Z, Izumi H, Kim SH, Mattison CP, Winey M, Fukasawa K (2007) Physical and functional interaction between mortalin and Mps1 kinase. Genes Cells 12:797–810
Kann O, Kovács R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292:C641–657
Karunakaran S, Saeed U, Mishra M, Valli RK, Joshi SD, Meka DP, Seth P, Ravindranath V (2008) Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. J Neurosci 28:12500–12509
Kaul SC, Wadhwa R, Matsuda Y, Hensler PJ, Pereira-Smith OM, Komatsu Y, Mitsui Y (1995) Mouse and human chromosomal assignments of mortalin, a novel member of the murine hsp70 family of proteins. FEBS Lett 361:269–272
Kaul SC, Deocaris CC, Wadhwa R (2007) Three faces of mortalin: a housekeeper, guardian and killer. Exp Gerontol 42:263–274
Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, Distefano LM, Nobrega JN (1992) Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem 59:776–779
Kitamura Y, Kosaka T, Kakimura JI, Matsuoka Y, Kohno Y, Nomura Y, Taniguchi T (1998) Protective effects of the antiparkinsonian drugs talipexole and pramipexole against 1-methyl-4-phenylpyridinium-induced apoptotic death in human neuroblastoma SH-SY5Y cells. Mol Pharmacol 54:1046–1054
Koppen M, Langer T (2007) Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol 42:221–242
Lanni C, Racchi M, Mazzini G, Ranzenigo A, Polotti R, Sinforiani E, Olivari L, Barcikowska M, Styczynska M, Kuznicki J, Szybinska A, Govoni S, Memo M, Uberti D (2008) Conformationally altered p53: a novel Alzheimer’s disease marker? Mol Psychiatry 13:641–647
Levy O, Malagelada C, Greene L (2009) Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 14:478–500
Lewis CM, Levinson DF, Wise LH, Delisi LE, Straub RE et al. (2003) Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet 73:34–48
Li HM, Niki T, Taira T, Iguchi-Ariga SM, Ariga H (2005) Association of DJ-1 with chaperones and enhanced association and colocalization with mitochondrial Hsp70 by oxidative stress. Free Radic Res 39:1091–1099
Liang ZQ, Li YL, Zhao XL, Han R, Wang XX, Wang Y, Chase TN, Bennett MC, Qin ZH (2007) NF-kappaB contributes to 6-hydroxydopamine-induced apoptosis of nigral dopaminergic neurons through p53. Brain Res 1145:190–203
Liu Y, Liu W, Song XD, Zuo J (2005) Effect of GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol Cell Biochem 268:45–51
Lodi R, Tonon C, Calabrese V, Schapira AH (2006) Friedreich’s ataxia: from disease mechanisms to therapeutic interventions. Antioxid Redox Signal 8:438–443
Lu WJ, Lee NP, Kaul SC, Lan F, Poon RT, Wadhwa R, Luk JM (2011) Induction of mutant p53-dependent apoptosis in human hepatocellular carcinoma by targeting stress protein mortalin. Int J Cancer (in press)
Ma Z, Izumi H, Kanai M, Kabuyama Y, Ahn NG, Fukasawa K (2006) Mortalin controls centrosome duplication via modulating centrosomal localization of p53. Oncogene 25:5377–5390
Massa SM, Longo FM, Zuo J, Wang S, Chen J, Sharp FR (1995) Cloning of rat grp75, an hsp70-family member, and its expression in normal and ischemic brain. J Neurosci Res 40:807–819
Mcculloch CC, Kay DM, Factor SA, Samii A, Nutt JG, Higgins DS, Griffith A, Roberts JW, Leis BC, Montimurro JS, Zabetia CP, Payami H (2008) Exploring gene-environment interactions in Parkinson’s disease. Hum Genet 123:257–265
Mcgrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76
Meriin AB, Sherman MY (2005) Role of molecular chaperones in neurodegenerative disorders. Int J Hyperthermia 21:403–419
Merims D, Freedman M (2008) Cognitive and behavioural impairment in Parkinson’s disease. Int Rev Psychiatry 20:364–373
Miller R, James-Kracke M, Sun G, Sun A (2009) Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34:55–65
Mizukoshi E, Suzuki M, Loupatov A, Uruno T, Hayashi H, Misono T, Kaul SC, Wadhwa R, Imamura T (1999) Fibroblast growth factor-1 interacts with the glucose-regulated protein GRP75/mortalin. Biochem J 343:461–466
Mizukoshi E, Suzuki M, Misono T, Loupatov A, Munekata E, Kaul SC, Wadhwa R, Imamura T (2001) Cell-cycle dependent tyrosine phosphorylation on mortalin regulates its interaction with fibroblast growth factor-1. Biochem Biophys Res Commun 280:1203–1209
Mogi M, Kondo T, Mizuno Y, Nagatsu T (2007) p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett 414:94–97
Morais VA, De Strooper B (2010) Mitochondria dysfunction and neurodegenerative disorders: cause or consequence. J Alzheimers Dis 20(Suppl 2):S255–263
Nair VD (2006) Activation of p53 signaling initiates apoptotic death in a cellular model of Parkinson’s disease. Apoptosis 11:955–966
Nair VD, Mcnaught KS, González-Maeso J, Sealfon SC, Olanow CW (2006) p53 mediates nontranscriptional cell death in dopaminergic cells in response to proteasome inhibition. J Biol Chem 281:39550–39560
Naylor DJ, Hoogenraad NJ, Høj PB (1996) Isolation and characterisation of a cDNA encoding rat mitochondrial GrpE, a stress-inducible nucleotide-exchange factor of ubiquitous appearance in mammalian organs. FEBS Lett 396:181–188
Nguyen HN, Lee MS, Hwang DY, Kim YK, Yoon DY, Lee JW, Yun YP, Lee MK, Oh KW, Hong JT (2007) Mutant presenilin 2 increased oxidative stress and p53 expression in neuronal cells. Biochem Biophys Res Commun 357:174–180
Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T (2005) Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer’s disease. FASEB J 19:255–257
Ordys BB, Launay S, Deighton RF, Mcculloch J, Whittle IR (2010) The role of mitochondria in glioma pathophysiology. Mol Neurobiol 42:64–75
Orr CF, Rowe DB, Halliday GM (2002) An inflammatory review of Parkinson’s disease. Prog Neurobiol 68:325–340
Osorio C, Sullivan PM, He DN, Mace BE, Ervin JF, Strittmatter WJ, Alzate O (2007) Mortalin is regulated by APOE in hippocampus of AD patients and by human APOE in TR mice. Neurobiol Aging 28:1853–1862
Patten DA, Germain M, Kelly MA, Slack RS (2010) Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis 20(Suppl 2):S357–367
Pennington K, Peng J, Hung CC, Banks RE, Robinson PA (2010) Differential effects of wild-type and A53 T mutant isoform of alpha-synuclein on the mitochondrial proteome of differentiated SH-SY5Y cells. J Proteome Res 9:2390–2401
Pilzer D, Saar M, Koya K, Fishelson Z (2010) Mortalin inhibitors sensitize K562 leukemia cells to complement-dependent cytotoxicity. Int J Cancer 126:1428–1435
Posner E (2008) Absence seizures in children. Clin Evid (Online) 2008:0317
Qu M, Zhou Z, Xu S, Chen C, Yu Z, Wang D (2011) Mortalin overexpression attenuates beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Brain Res 1368:336–345
Ran Q, Wadhwa R, Kawai R, Kaul SC, Sifers RN, Bick RJ, Smith JR, Pereira-Smith OM (2000) Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res Commun 275:174–179
Rintoul GL, Reynolds IJ (2010) Mitochondrial trafficking and morphology in neuronal injury. Biochim Biophys Acta 1802:143–150
Rocchi A, Pellegrini S, Siciliano G, Murri L (2003) Causative and susceptibility genes for Alzheimer’s disease: a review. Brain Res Bull 61:1–24
Ryu MJ, Lee C, Kim J, Shin HS, Yu MH (2008) Proteomic analysis of stargazer mutant mouse neuronal proteins involved in absence seizure. J Neurochem 104:1260–1270
Saito A, Maier CM, Narasimhan P, Nishi T, Song YS, Yu F, Liu J, Lee YS, Nito C, Kamada H, Dodd RL, Hsieh LB, Hassid B, Kim EE, González M, Chan PH (2005) Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol 31:105–116
Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, Kandel ER, Duff K, Kirkwood A, Shen J (2004) Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42:23–36
Schatz G (1995) Mitochondria: beyond oxidative phosphorylation. Biochim Biophys Acta 1271:123–126
Shan Y, Napoli E, Cortopassi G (2007) Mitochondrial frataxin interacts with ISD11 of the NFS1/ISCU complex and multiple mitochondrial chaperones. Hum Mol Genet 16:929–941
Shi M, Jin J, Wang Y, Beyer RP, Kitsou E, Albin RL, Gearing M, Pan C, Zhang J (2008) Mortalin: a protein associated with progression of Parkinson disease? J Neuropathol Exp Neurol 67:117–124
Shinbo Y, Taira T, Niki T, Iguchi-Ariga SM, Ariga H (2005) DJ-1 restores p53 transcription activity inhibited by Topors/p53BP3. Int J Oncol 26:641–648
Siesjö BK, Agardh CD, Bengtsson F (1989) Free radicals and brain damage. Cerebrovasc Brain Metab Rev 1:165–211
Silver I, Erecinska M (1998) Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv Exp Med Biol 454:7–16
Sirk D, Zhu Z, Wadia JS, Shulyakova N, Phan N, Fong J, Mills LR (2007) Chronic exposure to sub-lethal beta-amyloid (Abeta) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells. J Neurochem 103:1989–2003
Sklar P, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Verner A, Hudson TJ, Morley CP, Kennedy JL, Azevedo MH, Lander E, Daly MJ, Pato CN (2004) Genome-wide scan in Portuguese Island families identifies 5q31–5q35 as a susceptibility locus for schizophrenia and psychosis. Mol Psychiatry 9:213–218
Stambolic V, Macpherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW (2001) Regulation of PTEN transcription by p53. Mol Cell 8:317–325
Starkstein SE, Preziosi TJ, Berthier ML, Bolduc PL, Mayberg HS, Robinson RG (1989) Depression and cognitive impairment in Parkinson’s disease. Brain 112:1141–1153
Takano S, Wadhwa R, Yoshii Y, Nose T, Kaul SC, Mitsui Y (1997) Elevated levels of mortalin expression in human brain tumors. Exp Cell Res 237:38–45
Takano S, Wadhwa R, Mitsui Y, Kaul SC (2001) Identification and characterization of molecular interactions between glucose-regulated proteins (GRPs) mortalin/GRP75/peptide-binding protein 74 (PBP74) and GRP94. Biochem J 357:393–398
Tatton NA (2000) Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson’s disease. Exp Neurol 166:29–43
Taurin S, Seyrantepe V, Orlov SN, Tremblay TL, Thibault P, Bennett MR, Hamet P, Pshezhetsky AV (2002) Proteome analysis and functional expression identify mortalin as an antiapoptotic gene induced by elevation of [Na+]i/[K+]i ratio in cultured vascular smooth muscle cells. Circ Res 91:915–922
Trimmer PA, Smith TS, Jung AB, Bennett JP (1996) Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 5:233–239
Van Laar VS, Dukes AA, Cascio M, Hastings TG (2008) Proteomic analysis of rat brain mitochondria following exposure to dopamine quinone: implications for Parkinson disease. Neurobiol Dis 29:477–489
Van Laar VS, Mishizen AJ, Cascio M, Hastings TG (2009) Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol Dis 34:487–500
Van Os J, Kapur S (2009) Schizophrenia. Lancet 374:635–645
Voloboueva LA, Duan M, Ouyang Y, Emery JF, Stoy C, Giffard RG (2008) Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab 28:1009–1016
Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR, Mitsui Y, Kaul SC (1998) Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J Biol Chem 273:29586–29591
Wadhwa R, Sugihara T, Yoshida A, Nomura H, Reddel RR, Simpson R, Maruta H, Kaul SC (2000) Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res 60:6818–6821
Wadhwa R, Taira K, Kaul SC (2002) An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones 7:309–316
Wadhwa R, Ando H, Kawasaki H, Taira K, Kaul SC (2003a) Targeting mortalin using conventional and RNA-helicase-coupled hammerhead ribozymes. EMBO Rep 4:595–601
Wadhwa R, Yaguchi T, Hasan MK, Taira K, Kaul SC (2003b) Mortalin-MPD (mevalonate pyrophosphate decarboxylase) interactions and their role in control of cellular proliferation. Biochem Biophys Res Commun 302:735–742
Wadhwa R, Takano S, Taira K, Kaul SC (2004) Reduction in mortalin level by its antisense expression causes senescence-like growth arrest in human immortalized cells. J Gene Med 6:439–444
Wadhwa R, Takano S, Kaur K, Deocaris CC, Pereira-Smith OM, Reddel RR, Kaul SC (2006) Upregulation of mortalin/mthsp70/Grp75 contributes to human carcinogenesis. Int J Cancer 118:2973–2980
Warner TT, Schapira AH (2003) Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol 53(Suppl 3):S16–23 (discussion S23–25)
Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG (2009) Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab 29:365–374
Xu Z, Cawthon D, Mccastlain KA, Duhart HM, Newport GD, Fang H, Patterson TA, Slikker W, Ali SF (2005) Selective alterations of transcription factors in MPP+-induced neurotoxicity in PC12 cells. Neurotoxicol 26:729–737
Yoo JY, Ryu J, Gao R, Yaguchi T, Kaul SC, Wadhwa R, Yun CO (2010) Tumor suppression by apoptotic and anti-angiogenic effects of mortalin-targeting adeno-oncolytic virus. J Gene Med 12:586–595
Zyss D, Gergely F (2009) Centrosome function in cancer: guilty or innocent? Trends Cell Biol 19:334–346
Acknowledgments
J.J. was supported by funding from National Natural Sciences Foundation of China (30770760). T.J.C., J.G.H., and J.Z. have been supported in part by grants of the NIH (AG033398, ES004696-5897, ES016873, NS062684-6221, NS057567, and ES007032).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Jin, J., Zhang, J., Cook, T.J., Hoekstra, J.G. (2012). Mortalin in Neurological Diseases. In: Kaul, S., Wadhwa, R. (eds) Mortalin Biology: Life, Stress and Death. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3027-4_9
Download citation
DOI: https://doi.org/10.1007/978-94-007-3027-4_9
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-3026-7
Online ISBN: 978-94-007-3027-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)