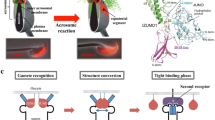
Abstract
In fertilization, two types of sex cells or gametes – a sperm and an egg – unite in a stepwise manner to form a mother cell, which is capable of developing naturally into a new individual. Notably, the “membrane fusion” that occurs intercellularly between a sperm and an egg is essential for fertilization. A sperm factor that is delivered into the egg cytoplasm through fusion serves to activate a signaling pathway; this leads to the resumption of meiosis in the egg. In mammals, sperm-egg fusion is partly mediated by two integral membrane proteins, sperm Izumo (Inoue et al. 2005) and egg cluster of differentiation 9 (CD9) (Kaji et al. 2000, Le Naour et al. 2000, Miyado et al. 2000), and the roles played by both are critical but yet unknown. A recent study (Miyado et al. 2000) showed that CD9-containing vesicles are released from wild-type eggs, and that these exosome-like vesicles induce fusion between sperm and CD9-null eggs in vitro, even though CD9-null eggs are highly refractory to sperm-egg fusion. This result provides compelling evidence for the crucial involvement of CD9-containing, fusion-facilitating vesicles in sperm-egg fusion and offers new insight into both gamete fusion and other membrane fusion events.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ADAM:
-
A disintegrin and metalloprotease
- CD:
-
Cluster of differentiation
- GM3:
-
Monosialo ganglioside 3
- EGFP:
-
Enhanced green fluorescent protein
- HIV:
-
Human immunodeficiency virus
- HSP:
-
Heat shock protein
- ICSI:
-
Intracytoplasmic sperm injection
- LEL:
-
Large extracellular loop
- MRP-1:
-
Motility-related protein 1
- M:
-
Microvilli
- PVS:
-
Perivitelline space
- Tspan:
-
Tetraspanin
- Z:
-
Zona pellucida
References
Akutsu H, Miura T, Machida M et al (2009) Maintenance of pluripotency and self-renewal ability of mouse embryonic stem cells in the absence of tetraspanin CD9. Differentiation 78:137–142
Almeida EA, Huovila AP, Sutherland AE et al (1995) Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 81:1095–1104
Andre M, Morelle W, Planchon S et al (2007) Glycosylation status of the membrane protein CD9P-1. Proteomics 7:3880–3895
Barraud-Lange V, Naud-Barriant N, Bomsel M et al (2007) Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J 21:3446–3449
Blobel CP, Wolfsberg TG, Turck CW et al (1992) A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356:248–252
Charrin S, Le Naour F, Labas V et al (2003) EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem J 373:409–421
Chen MS, Tung KS, Coonrod SA et al. (1999) Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc Natl Acad Sci USA 96:11830–11835
Chiu WH, Chandler J, Cnops G et al (2007) Mutations in the TORNADO2 gene affect cellular decisions in the peripheral zone of the shoot apical meristem of Arabidopsis thaliana. Plant Mol Biol 63:731–744
Clergeot PH, Gourgues M, Cots J et al (2001) PLS1, a gene encoding a tetraspanin-like protein, is required for penetration of rice leaf by the fungal pathogen Magnaporthe grisea. Proc Natl Acad Sci USA 98:6963–6968
Couzin J (2005) Cell biology: the ins and outs of exosomes. Science 308:1862–1863
Dandekar P, Aggeler J, Talbot P (1992) Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Hum Reprod 7:391–398
Evans JP (2001) Fertilin beta and other ADAMs as integrin ligands: insights into cell adhesion and fertilization. Bioessays 23:628–639
Garcia E, Pion M, Pelchen-Matthews A et al (2005) HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488–501
He ZY, Brakebusch C, Fassler R et al (2003) None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm-egg binding and fusion. Dev Biol 254:226–237
Hemler ME (2008) Targeting of tetraspanin proteins–potential benefits and strategies. Nat Rev Drug Discov 7:747–758
Hernandez LD, Hoffman LR, Wolfsberg TG et al (1996) Virus-cell and cell–cell fusion. Annu Rev Cell Dev Biol 12:627–661
Huang S, Yuan S, Dong M et al (2005) The phylogenetic analysis of tetraspanins projects the evolution of cell–cell interactions from unicellular to multicellular organisms. Genomics 86:674–684
Inoue N, Ikawa M, Isotani A et al (2005) The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434:234–238
Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112: 519–533
Kaji K, Oda S, Miyazaki S et al (2002) Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion. Dev Biol 247:327–334
Kaji K, Oda S, Shikano T et al (2000) The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 24:279–282
Kesimer M, Scull M, Brighton B et al (2009) Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J 23:1858–1868
Kopczynski CC, Davis GW, Goodman CS (1996) A neural tetraspanin, encoded by late bloomer, that facilitates synapse formation. Science 271:1867–1870
Lambou K, Tharreau D, Kohler A et al (2008) Fungi have three tetraspanin families with distinct functions. BMC Genomics 9:63
Le Naour F, Andre M, Boucheix C et al (2006) Membrane microdomains and proteomics: lessons from tetraspanin microdomains and comparison with lipid rafts. Proteomics 6:6447–6454
Le Naour F, Rubinstein E, Jasmin C et al (2000) Severely reduced female fertility in CD9-deficient mice. Science 287:319–321
Mi S, Lee X, Li X et al (2000) Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785–789
Miller BJ, Georges-Labouesse E, Primakoff P et al (2000) Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J Cell Biol 149:1289–1296
Miyado K, Yamada G, Yamada S et al (2000) Requirement of CD9 on the egg plasma membrane for fertilization. Science 287:321–324
Miyado K, Yoshida K, Yamagata K et al (2008) The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci USA 105:12921–12926
Miyake M, Koyama M, Seno M et al (1991) Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med 174:1347–1354
Moribe H, Yochem J, Yamada H et al (2004) Tetraspanin protein (TSP-15) is required for epidermal integrity in Caenorhabditis elegans. J Cell Sci 117:5209–5220
Okabe M, Cummins JM (2007) Mechanisms of sperm–egg interactions emerging from gene-manipulated animals. Cell Mol Life Sci 64:1945–1958
Rosen GD, Sanes JR, LaChance R et al (1992) Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 69:1107–1119
Rubinstein E, Ziyyat A, Prenant M et al (2006) Reduced fertility of female mice lacking CD81. Dev Biol 290:351–358
Runge KE, Evans JE, He ZY et al (2007) Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol 304:317–325
Saunders CM, Larman MG, Parrington J et al (2002) PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129:3533–3544
Shigeta M, Sanzen N, Ozawa M et al (2003) CD151 regulates epithelial cell–cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol 163:165–176
Silvie O, Rubinstein E, Franetich JF et al (2003) Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med 9:93–96
Simons M, Raposo G (2009) Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581
Tachibana I, Hemler ME (1999) Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J Cell Biol 146:893–904
Takeda Y, He P, Tachibana I et al (2008) Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease-like phenotype in mice. J Biol Chem 283:26089–26097
Takeda Y, Tachibana I, Miyado K et al (2003) Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol 161:945–956
Talbot P, DiCarlantonio G (1984) Ultrastructure of opossum oocyte investing coats and their sensitivity to trypsin and hyaluronidase. Dev Biol 103:159–167
Tanigawa M, Miyamoto K, Kobayashi S et al (2008) Possible involvement of CD81 in acrosome reaction of sperm in mice. Mol Reprod Dev 75:150–155
Todres E, Nardi JB, Robertson HM (2000) The tetraspanin superfamily in insects. Insect Mol Biol 9:581–590
Toshimori K, Saxena DK, Tanii I et al (1998) An MN9 antigenic molecule, equatorin, is required for successful sperm-oocyte fusion in mice. Biol Reprod 59:22–29
Trams EG, Lauter CJ, Salem N et al (1981) Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645:63–70
Valadi H, Ekstrom K, Bossios A et al (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Veneault-Fourrey C, Parisot D, Gourgues M et al (2005) The tetraspanin gene ClPLS1 is essential for appressorium-mediated penetration of the fungal pathogen Colletotrichum lindemuthianum. Fungal Genet Biol 42:306–318
Wiley RD, Gummuluru S (2006) Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci USA 103:738–743
Yamada M, Tamura Y, Sanzen N et al (2008) Probing the interaction of tetraspanin CD151 with integrin alpha 3 beta 1 using a panel of monoclonal antibodies with distinct reactivities toward the CD151-integrin alpha 3 beta 1 complex. Biochem J 415:417–427
Zhu GZ, Miller BJ, Boucheix C et al (2002) Residues SFQ (173-175) in the large extracellular loop of CD9 are required for gamete fusion. Development 129:1995–2002
Acknowledgments
We are grateful to Dr. Masaru Okabe, Dr. Kiyotaka Toshimori, Dr. Chizuru Ito, Dr. Naokazu Inoue, and Dr. Eisuke Mekada for their critical discussions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Kawano, N., Harada, Y., Yoshida, K., Miyado, M., Miyado, K. (2011). Role of CD9 in Sperm-Egg Fusion and Its General Role in Fusion Phenomena. In: Larsson, LI. (eds) Cell Fusions. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9772-9_7
Download citation
DOI: https://doi.org/10.1007/978-90-481-9772-9_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9771-2
Online ISBN: 978-90-481-9772-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)