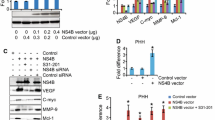
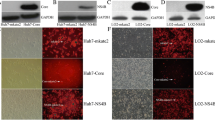
Summary
Hepatitis C virus (HCV) causes a persistent infection, chronic hepatitis, cirrhosis, and hepatocellular carcinoma. To explore the influence of HCV infection on hepatocytes, the effects of HCV proteins on intracellular signal transduction pathways, especially those related to apoptosis, fibrosis, and cell growth, were investigated. The effects of nine HCV proteins (core, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) on the CRE-, SRE-, NFKB-, AP-1-, SRF-, ISRE-, HSE-, GRE-, and p53-associated pathways were investigated by use of a reporter assay. The effects of core protein on apoptosis were examined by DNA laddering and Western blotting after induction of apoptosis by Fas stimulation. The possible mechanisms of anti-apoptotic effects of core protein were investigated by RNase protection assay and a reporter assay. The effects of HCV proteins on TGF beta production were determined by a reporter assay. Among seven HCV proteins investigated, core protein had the strongest influence on intracellular signaling, especially for the SRE-, AP-1-, NFKB-, and p53-associated pathways. Core protein promoted Bc1-xL expression through the mitogen-activated protein kinase (MAPK) pathway and inhibited apoptosis. Among HCV proteins, only core protein caused TGF beta promoter activity through the MAPK pathway. From these results, core protein was considered to regulate cell growth exquisitely in hepatocytes, inhibit apoptosis, and promote liver fibrosis directly without any inflammation or the mediation of any other cells. These functions may reflect the direct action of HCV proteins on the intracellular signal transduction pathways related to chronic hepatitis pathogenesis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (1989) Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244: 359–362
Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, Okudaira T, Teratani T, Tohgo G, Toda N, Ohashi M, Ogura K, Niwa Y, Kawabe T, Omata M (1995) Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology 22: 1027–1033
Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Shimotohno K (1991) Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci U S A 88: 5547–5551
Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM (1993) Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol 67: 1385–1395
Treisman R (1996) Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol 8: 205–215
Baeuerle PA, Henkel T (1994) Function and activation of NF-KB in the immune system. Annu Rev Immunol 12: 141–179
Karin M, Liu ZG, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246
Levine AJ (1997) p53, The cellular gatekeeper for growth and division. Cell 88:323–331
Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199
Kato N, Yoshida H, Ono-Nita SK, Kato J, Goto T, Otsuka M, Lan KH, Matsushima K, Shiratori Y, Ornata M (2000) Activation of intracellular signaling by hepatitis B and C viruses: C-viral core is the most potent signal inducer. Hepatology 32: 405–412
Yoshida H, Kato N, Shiratori Y, Otsuka M, Maeda S, Kato J, Ornata M (2001) Hepatitis C virus core protein activates nuclear factor KB-dependent signaling through tumor necrosis factor receptor-associated factor. J Biol Chem 276: 16399–16405
Kern SE, Pietenpol JA, Thiagalingam S, Seymour A, Kinzler KW, Vogelstein B (1992) Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256: 827–830
Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G, Iwanaga Y, Yamamoto N, Ohtani K, Nakamura M, Fujii M (1999) Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J Virol 73: 7981–7987
Kim SJ, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB (1989) Activation of the second promoter of the transforming growth factor-01 and phorbol ester occurs through the same target sequences. J Biol Chem 264: 19373–19378
Suzuki H, Chiba T, Kobayashi M, Takeuchi M, Furuichi K, Tanaka K (1999) IkappaBalpha ubiquitination is catalyzed by an SCF-like complex containing Skpl, cullin-1, and two F-box/WD40-repeat proteins, betaTrCP1 and betaTrCP2. Biochem Biophys Res Commun 256: 127 - 132, doi: 10.1006/bbrc.1999.0289
Sambrook J, Russel DW (2001) Molecular cloning, 3rd Edn. Cold Spring Harbor Laboratory Press. New York
Goto T, Kato N, Ono-Nita SK, Yoshida H, Otsuka M, Shiratori Y, Omata M (2001) Large isoform of hepatitis delta antigen activates serum response factor-associated transcription. J Biol Chem 275: 37311–37316
Marusawa H, Hijikata M, Chiba T, Shimotohno K (1999) Hepatitis C virus core protein inhibits Fas-and tumor necrosis factor alpha-mediated apoptosis via NF-KB activation. J Virol 73: 4713–4720
Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, Barr PJ (1995) Modulation of apoptosis by the widely distributed Bd-2 homologue Bak. Nature 374: 736–739
Otsuka M, Kato N, Taniguchi H, Yoshida H, Goto T, Shiratori Y, Ornata M (2002) Hepatitis C Virus core protein inhibits apoptosis via enhanced Bc1-xl expression. Virology 296: 84–93
Adams JM, Cory S (1998) The Bd-2 protein family: arbiters of cell survival. Science 281: 1322–1326
Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A (1996) Bc1–2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med 2: 80–86
You LR, Chen CM, Lee YHW (1999) Hepatitis C virus core protein enhances NF-kappaB signal pathway triggering by lymphotoxin-beta receptor ligand and tumor necrosis factor alpha. J Virol 73: 1672–1681
Tsuchihara K, Hijikata M, Fukuda K, Kuroki T, Yamamoto N, Shimotohno K (1999) Hepatitis C virus core protein regulates cell growth and signal transduction pathway transmitting growth stimuli. Virology 258: 100 - 107, doi: 10.1006/viro.1999.9694
Chinnaiyan AM, Orth K, O’Rourke K, Duan H, Poirier GG, Dixit VM (1996) Molecular ordering of the cell death pathway. Bd-2 and Bcl-xL function upstream of the CED-3like apoptotic proteases. J Biol Chem 271: 4573–4576
Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G (1999) NF-kappaB-mediated up-regulation of Bd-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci U S A 96: 9136–9141
Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M (1999) Tumor necrosis factor induces Bd-2 and Bd-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem 274: 8531–8538
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. (1998) NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680–1683
Hettmann T, DiDonato J, Karin M, Leiden JM (1999) An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med 189: 145–158
Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N (2000) MEK/ERK signaling pathway regulates the expression of Bd-2, Bc1-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem 79: 355–369
Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE (1989) An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244: 362–364
Roth S, Michel K, Gressner AM (1998) (Latent) transforming growth factor in liver parenchymal cells, its injury-dependent release, and paracrine effects on rat hepatic stellate cells. Hepatology 27: 1003–1012
Schmidt C, Royer C, Steffan AM, Labouret N, Caussin C, Navas MC, Jaeck D, Kirn A, Stollkeller F (1999) Interaction between human hepatic stellate cells and hepatitis C virus. Cells of the Hepatic Sinusoid 7: 249–250
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2003 Springer Japan
About this paper
Cite this paper
Otsuka, M., Kato, N., Taniguchi, H., Yoshida, H., Shiratori, Y., Omata, M. (2003). Signals Induced by HCV Proteins. In: Okita, K. (eds) HCV/Oxidative Stress and Liver Disease. Springer, Tokyo. https://doi.org/10.1007/978-4-431-67005-6_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-67005-6_4
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-67007-0
Online ISBN: 978-4-431-67005-6
eBook Packages: Springer Book Archive