Abstract
Wheat is cultivated across more land area than any other grain crops. Wheat cultivars are classified as two general types: winter wheat with variable low temperature requirement for a proper flowering time (vernalization) and spring wheat without the requirement, based on their qualitative vernalization requirement. Winter wheat cultivars are classified as three types, weak winter, semi-winter and strong winter, according to their quantitative vernalization requirement to reach a vernalization saturation point or achieve the maximum vernalization effect. Three vernalization genes, VRN1, VRN2, and VRN3, were cloned using a positional cloning approach and a one-gene model of qualitative variation in vernalization requirement between spring and winter wheat. A major gene for the vernalization requirement duration in winter wheat was mapped using a population of recombinant inbred lines (RILs) that were generated from two winter wheat cultivars, ‘Jagger’ and ‘2174’. Furthermore, the cloning population was developed using a RIL to backcross with 2174, which was segregated in a 3:1 ratio of the early flowered plants and the late flowered plants after the population was vernalized for 3 weeks. The wild type Jagger vrn-A1a allele for less vernalization was dominant over the 2174 vrn-A1b allele for more vernalization, and the two alleles encoded the vrn-A1 proteins with two point mutations. A third haplotype with one of the point mutations was found in common wheat. Gene markers were developed to direct breeding of semi-winter and strong winter wheat cultivars to adapt to different geographical areas and changing climates.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Wheat (Triticum aestivum, 2n = 6x = 42, AABBDD) is one of the mostly cultivated crops in different geographical areas in the world. Wheat is traditionally divided into two types, winter wheat with vernalization requirement and spring wheat without this requirement. Vernalization is an exposure of the plant to a few weeks of low temperature in order to accelerate its ability to transition from vegetative to reproductive development, providing an adaptive mechanism for winter wheat to synchronize its developmental transition with seasonal changes in temperature (Rawson et al. 1998). Winter wheat is sown in autumn, whereas spring wheat is sown in autumn or spring season. Advances in understanding the genetic basis and molecular mechanisms of winter wheat development are critically important to ensure that winter wheat flowers at a proper time.
Vernalization has significant effects at typically 2–10 °C, with dramatic decline at temperatures above 11 °C and an apparent loss of effects above 18 °C (Brooking 1996). Based on their various vernalization requirement durations with a combination of the amplitude and duration of low temperatures in different geographic areas, winter wheat cultivars are typically categorized into three types: a weak winter type that is stimulated to flower by brief exposure to low temperature, a semi-winter type that requires 2–4 weeks of cold exposure for flowering, and a strong winter type that needs 4–6 weeks of cold exposure (Crofts 1989). The winter wheat cultivar Yeoman reportedly requires up to 12 weeks at low temperature to attain a vernalization saturation point (Berry et al. 1980).
Recent studies suggest that global climate will inevitably produce significant changes in air temperatures (Körner and Basler 2010). Average global surface air temperature rose 0.5 °C in the twentieth century or 4–7 °C in the past million years between the ice ages and the warm interglacial periods (Solomon et al. 2007). The global mean temperature is projected to continue its increase by roughly 3 °C (Kerr 2007) or 5 °C by the end of the twenty-first century (Semenov and Halford 2009). Various crops, requiring specific environmental cues for growth and development, will inevitably respond differently to changing climate (Craufurd and Wheeler 2009; Lanning et al. 2010). As various simulation models have shown, winter wheat is more vulnerable to changing climate due to its higher sensitivity to temperatures for proper flowering time and successful grain reproduction (Morison and Long 1995). Higher temperatures in a winter season will lead to insufficient or failed vernalization hence delayed reproductive development for successful grain production of winter wheat.
Three genes controlling vernalization requirement in wheat were cloned, including VRN1 (Yan et al. 2003), VRN2 (Yan et al. 2004), and VRN3 (Yan et al. 2006). VRN1 is an orthologue of AP1 in Arabidopsis, and a dominant Vrn1 allele originated from mutations in the promoter or first intron of a recessive wild type vrn1 gene. VRN2 encodes a novel transcription factor containing a Zinc finger and a CCT domain, and a recessive vrn2 allele had a point mutation in coding region resulting in an alteration of an amino acid at the CCT domain or complete deletion, compared to the dominant allele as a wild type. VRN3 is an orthologue of Arabidopsis FT, and allelic variation at VRN3 is related with mutations in its promoter or intron one. VRN1 and VRN3 were cloned in populations that were segregated as a 3:1 ratio of early and late flowering plants, indicating that both VRN1 and VRN3 are promoters of flowering. VRN2 was cloned in a population that was segregated as a 1:3 ratio of early and late flowering plants, indicating that this gene is a repressor of flowering. Heading date in each of these populations was controlled by one gene, whereas the other two were fixed at the same allele.
In our previous studies on winter wheat, we found that the vrn-A1 locus was associated with variation in the stem elongation in the winter wheat Jagger × 2174 RIL population (Chen et al. 2009), and this locus influenced subsequent timing of heading and physiological maturity when characterized in the field for 3 years (Chen et al. 2010). This locus is very sensitive to temperature change and bears close association with variation in development in the winter wheat population that may be caused by vernalization requirement duration across years. The present study aimed to genotype wheat germplasm and to establish genetic models accounting for strong winter, semi-winter, and weak winter wheat cultivars.
Materials and Methods
In our previous study, we found a C/T polymorphism in exon 4 that is responsible for the amino acid change at Leu117/Phe117 in TaVRN-A1 between the Jagger allele and the 2174 allele. A PCR marker for this polymorphism showed an association with developmental variation in the Jagger × 2174 RIL population tested in the field (Chen et al. 2009). Primers used for the SNP in exon 4 are vrn-A1F4F 5′-CAACTTGTTTGGGACTAAAGGC-3′ and vrn-A1F4R 5′-CTGCAACTCCTTGAGATTCAAAG-3′. PCR was performed for 40 cycles (90 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s per cycle) followed by a 10-min final extension at 72 °C. PCR products were digested with DpnII and run on a 1 % agarose gel.
In this study, we found that there was another SNP in exon 7, and we developed the PCR marker for the SNP in exon 7 to screen wheat germplasm available in our laboratory. The hexaploid wheat cDNA sequences in GenBank for each of three homoeologous VRN1 genes were divided into three groups, and genomic DNA sequences for each group were derived from the wheat genome sequence database (http://www.cerealsdblished.uk.net/). Specific primers for each homoeologous VRN1 were designed. Chinese Spring nullitetrasomic lines, N5AT5D, N5BT5D or N5DT5B missing each of three homoeologous group five chromosomes were used to determine specificity of the primers. Primers vrn-A1F7B (5′-GTGGAGAAGCAGAAGGCGCATG-3′) and vrn-A1R7 (5′-CCGACAGAACTGCATAGAGACC-3′) were designed to detect the SNP encoding A180/V180 between the Jagger vrn-A1a allele and 2174 vrn-A1b allele. The two primers amplified a 221 bp fragment using an annealing temperature of 55 °C and extension time for 1 min. The PCR products digested with restriction enzyme SphI were run on a 1 % agarose gel, showing polymorphic bands between the vrn-A1a allele (199 bp) and vrn-A1b allele (221 bp).
Results and Discussion
The vrn-A1 Gene Controlling Vernalization Requirement Duration in Winter Wheat Cultivars
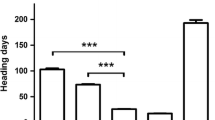
Jagger required 3 weeks to reach the maximum vernalization effect on flowering, whereas 2174 required 6 weeks to reach the maximum vernalization effect on flowering under the same condition (Li et al. 2013). We generated a BC1F2 population using RIL23 to backcross with 2174. When vernalized for 3 weeks, heading date of 90 F2 plants showed a clear segregation. On average, 24 plants homozygous for the Jagger vrn-A1a allele headed at 110 days after planting, 20 plants homozygous for the 2174 vrn-A1b allele headed at 138 days, and 46 plants heterozygous at VRN-A1 headed at 118 days (Fig. 13.1a). The 70 plants either homozygous or heterozygous for the Jagger vrn-A1a allele for early heading showed a significant difference from the 20 plants homozygous for the 2174 vrn-A1b allele for late heading (p < 0.001). The observed segregation ratio between the earlier heading and later heading groups was not significantly different from a 3:1 ratio (X 2 = 0.37, df = 1, p = 0.54) and fit a one-gene model.
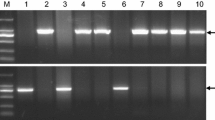
Allelic variation and genetic effects of vrn-A1. (a) The segregation of heading date in plants carrying different vrn-A1 alleles in a BC1F2 population. vrn-A1a for the Jagger allele and vrn-A1b for the 2174 allele. (b) PCR marker for the SNP in exon 4 of vrn-A1 using primers vrn-A1F4F and vrn-A1F4R. (c) PCR marker for the SNP in exon 7 of vrn-A1 using primers vrn-A1F7F and vrn-A1F7R
A Critical Point Mutation in vrn-A1 at the Protein Level
We used the positional cloning strategy to prove that quantitative vernalization requirement in winter wheat is controlled by vrn-A1 at the protein level (Li et al. 2013). There were 29 SNPs in vrn-A1 between the Jagger allele (11,922 bp, JQ915055) and the 2174 allele (11,921 bp, JQ915056), including 464 bp from the start codon for translation, the complete gene from the start codon and the stop codon, 41 bp after the stop codon. These SNPs were confirmed by sequencing two independent PCR products or digesting PCR products using restriction enzymes wherever appropriate. No difference was observed between the Jagger vrn-A1a allele and 2174 vrn-A1b allele in the previously identified regulatory sites in the promoter or intron one that accounted for allelic variation between the winter vrn-A1 allele and the spring Vrn-A1 allele.
There were two SNPs in exon 4 and exon 7, both of which resulted in an altered amino acid in the conserved domain between the Jagger and 2174 alleles. An amino acid Ala180 encoded in exon 7 in the Jagger vrn-A1a allele controlling less vernalization or early flowering was mutated to Val180 in the 2174 vrn-A1b allele. The Ala180/Val180 substitution accounted for the differential interactions of vrn-A1a and vrn-A1b with TaHOX1 in pull-down assays and protein immune-precipitation analyses (Li et al. 2013).
A Novel Haplotype of vrn-A1 at the Protein Level
The vrn-A1 sequences were used to search GenBank expressed sequence tags (EST) databases. Diploid wheat T. urartu (DQ291016) and tetraploid wheat T. turgidum ssp. durum (AY747598) have the same sequences at SNPs at both exon 4 and exon 7 as Jagger (Fig. 13.2), suggesting that the vrn-A1a allele in Jagger is the wild type. This result was confirmed using a PCR marker for another two accessions of diploid wheat T. urartu and 11 accessions of tetraploid wheat T. turgidum ssp. durum, ssp. dicocoides, and ssp. dicoccum (Table 13.1).
Multiple sequence alignment of the VRN-A1 genes in different wheat species. The sequences of Jagger and 2174 were obtained in this study, and other sequences were from GenBank. T. urartu: DQ291016; T. durum: Langdon, AY747598; CS: Chinese Spring, AM502869; Norstar, DY761363; Recital: CD872434, CD936812; IL369: AY747599; Nongda 3338: DQ512342. The two SNPs in exons 4 and 7 resulting in alternation of amino acids are highlighted. N indicates sequences between exon 4 and exon 7
There are five hexaploid wheat cultivars, which VRN-A1 sequences are deposited in GenBank (Fig. 13.2). Chinese Spring was found to carry the vrn-A1a allele. Two winter wheat cultivars, Norstar and Recital, were found to carry the vrn-A1b allele. Norstar carrying the vrn-A1b allele was one of the most cold-hardy wheat cultivars. Interestingly, another two cultivars, IL369 and Nongda 3338, were found to have a novel haplotype at the protein level. The two cultivars have the mutation at Ala180/Val180 but not at Leu117/Phe117. Therefore, the third haplotype differing from the Jagger vrn-A1a allele and the 2174 vrn-A1b allele was referred to the vrn-A1c allele. These observations suggested that mutations from the wild type vrn-A1a allele to the vrn-A1b allele occurred twice, and the vrn-A1c allele is an intermediate type between them. The mutation in exon 7 should occur preceded to the mutation in exon 7 of vrn-A1.
The disassociation between Leu117/Phe117 and Ala180/Val180 suggested that the previously reported marker for Leu117/Phe117 (Fig. 13.1b) (Chen et al. 2009) cannot be used for the Ala180/Val180 mutation found in this study. A new marker has been developed for Ala180/Val180 PCR markers for vrn-A1 were developed to distinguish the SNP in exon 7 (Fig. 13.1c) between the Jagger and 2174 alleles.
Diverse VRN-A1 Proteins in Winter Wheat and Spring Wheat Cultivars
The two PCR markers, the SNPs in exon 4 and exon 7 between the Jagger and 2174 alleles, were used to determine the genotypes of wheat germplasm (Table 13.1). Among 73 cultivars of hexaploid wheat, 18 cultivars carry the vrn-A1a allele, 50 cultivars carry the vrn-A1b allele, and 5 cultivars carry the vrn-A1c allele. The higher frequency of the vrn-A1b allele in contemporary wheat cultivars indicated that breeders in the southern Great Plains have inadvertently selected this allele, contributing to delay stem elongation and extend the vegetative phase of the dual purpose wheat to produce more biomass for cattle.
Five cultivars carrying the vrn-A1c allele, Jaypee (VA), Pio 26R61 (NY), OK Bullet, IL369, and Nongda 3338, do not have the same pedigree, indicating that the vrn-A1c allele in these cultivars has evolved independently. The possibility that these cultivars have the same tetraploid wheat donor carrying the vrn-A1c allele cannot be excluded, though none of the tetraploid wheat accessions tested in this study carried the vrn-A1c allele.
When vrn-A1 acts as a protein form, it should appear as a VRN-A1-TaHOX1 protein complex because of a direct binding between them. VRN-A1 protein and TaHOX1 protein should also have direct interaction in spring wheat cultivars. It was found that both vrn-A1a and vrn-A1b existed in spring wheat cultivars. Nine spring wheat cultivars in the CAP group carry the vrn-A1a allele, including UC1110 (CA), ID0556 and Zak (ID), McNeal and Thatcher (MT), Louise and Panawawa (WA), GRN*5/ND614-A (MN), Jupeteco (TX); whereas four spring wheat cultivars carry the vrn-A1b allele, including CIMMYT-2 (PI610750) (CA), OR9900553 (OR), NY18/ Clark’s Cream 40–1 (MN), and Weebill (TX).
Allelic variation in the dominant Vrn-A1 locus also indicated pleiotropic genetic effects in spring wheat cultivars (Blake et al. 2009; Santra et al. 2009; Zhang et al. 2008), supporting that the VRN-A1 gene has different mechanisms in controlling wheat development. There were three CAP populations, UC1110 and CIMMYT-2 (PI610750) (CA), GRN*5/ND614-A and NY18/ Clark’s Cream 40–1 (MN), and Jupeteco and Weebill (TX) that have different VRN-A1a and VRN-A1b forms. It would be interesting to investigate how allelic variation in VRN-A1 is associated with variation in development in spring wheat populations. Similarly, Jaypee (VA) and Pio 26R61 (NY), which have a VRN-A1c form, can be used to investigate effects of a single point substitution Ala180/Val180 on development in wheat.
Application of Multiple Molecular Markers for VRN-A1
Molecular breeding is a new strategy that uses contemporary methods of molecular genetics and genomic sequencing to assist in all steps throughout the procedure of conventional breeding. Most of the molecular markers currently being applied in breeding are SSR (simple sequence repeat) markers due to their relative simplicity for breeding applications. However, the ‘repeat’ feature of a SSR marker results in multiple and inconsistent locations of the same marker among divergent wheat cultivars. SNP markers would provide more powerful tools to assist selection in breeding (Akhunov et al. 2009). In most cases, however, a SNP marker is derived from the sequence of an EST that is randomly distributed among genomes; thus a SNP marker may not associate with a given trait. A gene marker (perfect marker) that is developed for the specific regulatory site of a functional gene can provide the ultimate resolution to select for a given trait.
Four markers have been developed for allelic variation in VRN-A1. It is not yet known if the allelic variation in exon 4 is associated with any trait, though the point mutation occurred in the K-box, a critical domain for protein-protein interaction. Two markers for allelic variation in the promoter (Yan et al. 2004) and intron one (Fu et al. 2005) should be used to distinguish between spring wheat and winter wheat, whereas the marker for the SNP intron 7 should be used to distinguish semi-winter and strong winter wheat cultivars.
References
Akhunov E, Nicolet C, Dvorak J (2009) Single nucleotide polymorphism genotyping in polyploid wheat with the illumina GoldenGate assay. Theor Appl Genet 119:507–517
Berry GJ, Salisbury PA, Halloran GM (1980) Expression of vernalization genes in near-lsogenic wheat lines: duration of vernalization period. Ann Bot 46:235–241
Blake NK, Lanning SP, Martin JM et al (2009) Effect of variation for major growth habit genes on maturity and yield in give spring wheat populations. Crop Sci 49:1211–1220
Brooking IR (1996) Temperature response of vernalization in wheat: a developmental analysis. Ann Bot 78:507–512
Chen YH, Carver BF, Wang SW et al (2009) Genetic loci associated with stem elongation and winter dormancy release in wheat. Theor Appl Genet 118:881–889
Chen Y, Carver BF, Wang S et al (2010) Genetic regulation of developmental phases in winter wheat. Mol Breed 118:1339–1349
Craufurd PQ, Wheeler TR (2009) Climate change and the flowering time of annual crops. J Exp Bot 60:2529–2539
Crofts HJ (1989) On defining a winter wheat. Euphytica 44:225–234
Fu D, Szucs P, Yan L, Helguera M, Skinner J, Hayes P, Dubcovsky J (2005) Large deletions in the first intron of the VRN-1 vernalization gene are associated with spring growth habit in barley and polyploid wheat. Mol Gen Genomics 273:54–65
Kerr RA (2007) Climate change: global warming is changing the world. Science 316:188–190
Korner C, Basler D (2010) Phenology under global warming. Science 327:1461–1462
Lanning SP, Kephart K, Carlson GR et al (2010) Climatic change and agronomic performance of hard red spring wheat from 1950 to 2007. Crop Sci 50:835–841
Li G, Yu M, Fang T et al (2013) Vernalization requirement duration in winter wheat is controlled by TaVRN-A1 at the protein level. Plant J 76:742–753
Morison JIL, Long SP (1995) Wheat growth under global environmental change-an introduction. Glob Chang Biol 1:383–384
Rawson HM, Zajac M, Penrose LDJ (1998) Effect of seedling temperature and its duration on development of wheat cultivars differing in vernalization response. Field Crops Res 57:289–300
Santra DK, Santra M, Allan RE et al (2009) Genetic and molecular characterization of vernalization genes Vrn-A1, Vrn-B1, and Vrn-D1 in spring wheat germplasm from the Pacific Northwest region of the USA. Plant Breed 128:576–584
Semenov MA, Halford NG (2009) Identifying target traits and molecular mechanisms for wheat breeding under a changing climate. J Exp Bot 60:2791–2804
Solomon S, Qin D, Manning M, Marquis M, Averyt K, Tignor MMB, LeRoy MH, Chen Z (2007) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovermental panel on climate change. Cambridge University Press, New York
Yan L, Loukoianov A, Tranquilli G et al (2003) Positional cloning of wheat vernalization gene VRN1. Proc Natl Acad Sci 100:6263–6268
Yan L, Loukoianov A, Tranquilli G et al (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303:1640–1644
Yan L, Fu D, Li C et al (2006) From the cover: the wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci 103:19581–19586
Zhang XK, Xia XC, Xiao YG et al (2008) Allelic variation at the vernalization genes Vrn-A1, Vrn-B1, Vrn-D1 and Vrn-B3 in Chinese common wheat cultivars and their association with growth habit. Crop Sci 48:458–470
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Yan, L., Li, G., Yu, M., Fang, T., Cao, S., Carver, B.F. (2015). Genetic Mechanisms of Vernalization Requirement Duration in Winter Wheat Cultivars. In: Ogihara, Y., Takumi, S., Handa, H. (eds) Advances in Wheat Genetics: From Genome to Field. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55675-6_13
Download citation
DOI: https://doi.org/10.1007/978-4-431-55675-6_13
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55674-9
Online ISBN: 978-4-431-55675-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)