Abstract
This article introduces Kihara’s main achievements in wheat cytogenetics and the succeeding developments in a few fields of wheat cytogenetics, which were founded by Kihara. Following the discovery of polyploidy in wheat by Sakamura (Bot Mag (Tokyo) 32:150–153, 1918), Kihara established the cytogenetics of interploid hybrids, clarifying the meiotic chromosome behavior as well as the chromosome number and genome constitution of their progeny, based on which Kihara formulated the concept of genome. Here, evidence supporting his recognition of the genome as a functional unit is presented. Kihara proposed the methodology for genome analysis and determined the genome constitution of all Triticum and Aegilops species. Ohta re-evaluated the genome relationships among the diploid species, using the B-chromosomes of Ae. mutica. After completing the genome analyses, Kihara’s interest was shifted to the genome-plasmon interaction that led to the discovery of cytoplasmic male sterility in wheat. Using the nucleus substitution method elaborated by Kihara, we carried out plasmon analysis of Triticum and Aegilops species. We classified their plasmons into 17 major types and 5 subtypes and determined the maternal and paternal lineages of all polyploid species. An alloplasmic line, (caudata)-Tve, retained male sterility induction and germless grain production for 60 generations of backcrosses with wheat pollen. We are trying reconstruction of the Ae. caudata plant from the genome of its native strain and the caudata plasmon in the alloplasmic wheat. Two groups of Kihara’s school reported paternal transmission of the mtDNA sequences in alloplasmic wheats. Their findings are incompatible with the genetic autonomy of the plasmon, casting a new challenge to the genome-plasmon interaction.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Aegilops
- B-chromosomes
- Genome analysis
- Genome concept
- Maternal lineage
- Plasmon analysis
- Plasmon autonomy
- Polyploids
- Wheat
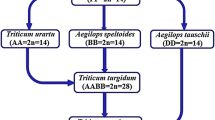
Discovery of Polyploidy and Cytogenetics of Interploid Hybrids in Wheat
Sakamura (1918) who was a graduate student at the Faculty of Agriculture, Hokkaido University, studied both root-tip mitosis and meiosis in PMC’s of the following eight Triticum species; T. aestivum, T. compactum, T. spelta, T. turgidum, T. durum, T. polonicum, T. dicoccum, and T. monococcum, finding 2n = 14 for T. monococcum, 2n = 28 for T. turgidum, T. durum, T. polonicum and T. dicoccum, and 2n = 42 for T. aestivum, T. compactum and T. spelta. This result led him to discover a polyploid series of the diploid, tetraploid, and hexaploid in wheat, with the basic chromosome number of x = 7. Sakamura planned a further study on chromosome numbers of the offspring of the hybrids between different ploidies. In 1917 he made crosses between 4x and 6x wheat in three combinations. At this point, Sakamura was informed from the Ministry of Education, Japan, to go abroad for advanced study. He asked Kihara who had just enrolled in the graduate school to succeed his wheat research and handed the 5x hybrids to Kihara (1951). Because of some delay in departure, Sakamura was able to see Kihara’s first slide of PMC’s of the 5x hybrid, and gave a few minutes advice that determined Kihara’s later career as the wheat researcher.
Kihara (1924) analyzed the meiotic chromosome behavior of the three 5x hybrids of Sakamura and two 3x hybrids that he produced. The modal meiotic chromosome configurations of the 3x and 5x hybrids were 7” + 7’ and 14” + 7’, respectively (Kihara 1924, 1930), based on which he assigned genome formulae AA to the diploid, AABB to the tetraploid, and AABBDD to the hexaploid wheat. Later Kihara obtained a new tetraploid wheat, T. timopheevi, and analyzed the meiotic chromosome behaviors of its hybrids with one diploid and two tetraploid Triticum species. From the results, he designated genome formula AAGG to this wheat (Lilienfeld and Kihara 1934).
Kihara’s Genome Concept and Supporting Evidence
Winkler (1920) proposed the term ‘genome’ for the haploid set of chromosomes. Due to the discovery of polyploidy, this definition required modification, because their gametes contain two or more chromosome sets. Kihara and Lilienfeld (1932) and Kihara (1982) defined the genome concept as follows: (1) Homologous chromosomes have homologous loci identical in sequence as well as in distance. Therefore, when two genomes are homologous, an exchange of homologous partners causes no physiological damage to either the gametes or the zygotes. Non-homologous chromosomes have different loci or the same loci different in sequence or in distance. As a consequence, the homologous chromosomes can synapse in the meiotic prophase, forming bivalents in the metaphase I (MI) by exchanging their homologous parts. On the contrary, non-homologous chromosomes fail to pair, becoming univalents in MI. (2) Genome has no homologous chromosomes within it. Consequently, a zygote having two homologous genomes forms x pairs of bivalents with no univalents, whereas that with two non-homologous genomes forms 2x univalents with no bivalents in meiosis. The homologous vs. non-homologous relationship between two genomes can be determined by the number of bivalents formed in meiosis. (3) Genome is a functional unit of life. The deletion of a chromosome or a part of it from a genome causes the loss of life or, at least, a significant loss of functions of the gamete and zygote. In essence, Kihara was first to define the homology of chromosomes by their meiotic behavior, based on which he defined genome homology, and proposed its functional role in life.
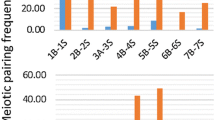
Supportive evidence of the functional role of genome in gametic and zygotic development was obtained from the fertility of gametes and the viability of progenies of the 5x hybrids, respectively. Fertility of the female and male gametes of the 5x hybrids was studied by Kihara and Wakakuwa (1935) and Matsumura (1936, 1940). The transmission rate of a D-genome chromosome was 0.440 when the 5x hybrid was backcrossed as female to the 4x parent, and 0.673 when the 5x hybrid was backcrossed as pollen parent to the 4x wheat. Based on these univalent transmission rates, the fertility rates of female and male gametes having zero to seven D-genome chromosomes of the 5x hybrid when backcrossed as female or male parent to the 4x wheat were estimated, and compared to the observed frequencies. The female gametes of the 5x hybrid having no D-genome chromosomes or the complete set of D genome chromosomes took part in fertilization in 3.8 or 5.6 times higher frequencies than the expected ones, whereas those with one to five D genome chromosomes showed fertility rates nearly equal to or lower than the expected frequencies (Fig. 1.1a). Similarly, the male gametes of the 5x hybrid having no D genome chromosomes or the complete set of D genome chromosomes took part in fertilization in 391 or 5.7 times higher frequencies, respectively, than expected, whereas those with two to six D genome chromosomes showed fertility rates nearly equal to or lower than the expected frequencies (Fig. 1.1b). These results showed that complete missing or presence of the complete D genome guaranteed both sexes of the gametes for high ability of fertilization. The effect of the genome completeness on the viability of sporophytes was traced for four generations of self-pollination of the 5x hybrid (Kihara 1924). The pedigree was converged at two extremes, 2n = 28 with 14” and 2n = 42 with 21”, one exception being the stable 2n = 40 (20”) progenies called D-dwarfs. This convergence of the pedigrees to 2n = 28 or 2n = 42 plants demonstrated importance of the genome completeness for the continuation of life even in the hexaploid wheat.
Genome Analysis and a Re-evaluation on Genome Homology
Determination of the genome homology is based on the meiotic chromosome pairing between two genomes in comparison. Kihara (1930) illustrated the schemes for genome analysis of an auto- and allotetraploid. Before their application, we need to have a set of diploids, each having a genome different from the others, which he called genome analyzers. When the tetraploid is an autotetraploid with A genome, its hybrid with the A genome analyzer forms x”’, whereas the F1’s with other analyzers form x” + x’, from which results genome constitution of the tetraploid is determined as AAAA. If the tetraploid is an allotetraploid with AABB genomes, its F1 hybirds with the A and B genome analyzers form x” + x’, whereas the F1’s with other analyzers form 3x’, thus genome constitution of the tetraploid is confirmed to be AABB. Kihara and his collaborators determined the genome constitutions of all Triticum and Aegilops species (Kihara 1924, 1945; Kihara and Tanaka 1970; Lilienfeld 1951).
After Kihara’s genome analytical works, several genetic factors became known to influence the meiotic chromosome pairing: They are a suppressor, Ph1, of the homoeologous chromosome pairing, an enhancer of the homoeologous pairing in Ae. speltoides and a suppressor of the homoeologous pairing in B-chromosomes. To distinguish between the homologous and homoeologous pairing, using B-chromosomes of Ae. mutica, Ohta (1995) produced hybrids between eight diploid species and an Ae. mutica strain having the B-chromosomes, selecting hybrids with zero, one or two B-chromosomes. The presence of two B-chromosomes did not affect the number of bivalents in Ae. mutica itself. The T genome of Ae. mutica was highly homologous to S genome of Ae. speltoides and D genome of Ae. squarrosa, forming five bivalents in the presence of two B’s, whereas it was non-homologous to A, C, M, Sb and Sl genomes of the respective species, forming no or one bivalent with two B’s. This type of research should be extended to polyploid species for re-evaluation of their genome relationships to the diploid species.
Plasmon Analysis as the Counter Part of Genome Analysis
Later, Kihara’s interest shifted to the genome-plasmon interaction. He produced an alloplasmic line of a common wheat, T. aestivum var. erythrospermum (abbrev. ‘Tve’) by repeated backcrosses of the F1 hybrid, Ae. caudata var. polyathera x Tve, with Tve as the recurrent pollen parent. This alloplasmic line, designated by (caudata)-Tve, expressed male sterility in its SB3 and later backcross generations, leading Kihara to discover the cytoplasmic male sterility in wheat (Kihara 1959).
The pioneering works of Kihara and others suggested the presence of plasmon diversity in the Triticum-Aegilops complex. There were three research groups actively working on the plasmon diversity in wheat and its related genera: Maan and Lucken in the North Dakota State Univ., USA, Panayotov and Gotsov in the Wheat and Sunflower Institute, Bulgaria, and Suemoto and Tsunewaki in Kyoto University, Japan. In an international cooperative work, we compared plasmons that were independently introduced by these groups into their own wheat stocks (Mukai et al. 1978). This work showed the wide scope of plasmon diversity in this complex. I obtained 7 plasmons from Maan, 8 plasmons from Panayotov and 15 plasmons from other researchers to enrich our plasmon collection, totaling 46 plasmons, including 16 of our own.
In 1963, I initiated a program to produce alloplasmic lines using a set of 12 common wheat genotypes as the alloplasmon recipients, whose list and reasons of selection are given elsewhere (Tsunewaki et al. 1996). The aim was 10 backcross generations of each alloplasmic line to recover the tester’s genotype in 99.9 % purity. The total number of the alloplasmic lines in all combinations between the 12 wheat genotypes and 46 plasmons amounts to 552 NC hybrids, all of which reached SB10 or later backcross generation by 1997. A field test of all alloplasmic and 12 euplasmic lines was carried out in the crop season of 1992–1993. By that time, 87 % of the alloplasmics reached at the SB10 or later backcross generation. With all lines, 14 vegetative and 8 reproductive characters were observed. The genetic relationships between the 47 plasmons were analyzed (Tsunewaki et al. 2002). With the same plasmons, RFLP analyses of ct and mtDNAs were carried with 13 and 3 restriction enzymes, respectively (Ogihara and Tsunewaki 1988; Wang et al. 2000). Phylogenetic trees depicted from the data on the organellar DNA polymorphisms revealed molecular differentiation between the plasmons in Triticum-Aegilops complex. Combining the phenotypic effects on wheat characters and organellar DNA differences revealed by the RFLP analyses, 47 plasmons of this complex were classified into 18 major types and five subtypes (Tsunewaki et al. 2002). The genome and plasmon analyses together clarified the maternal and paternal lineages of all Triticum and Aegilops species (Fig. 1.2; Tsunewaki 2009).
Phylogenetic relationships between the diploid, tetraploid and hexaploid species based on their genome-plasmon constitutions (After Tsunewaki 2009). Inner and outer circle: genome and plasmon symbol, respectively. Modified genome: underlined
Persistence of Genetic Effects of Ae. caudata Plasmon on Wheat Phenotypes
Kihara crossed in 1949 Ae. caudata as female to a common wheat, Tve, and the F1 hybrid and its progenies were successively backcrossed with the pollen of Tve until SB16 (Kihara 1959; unpubl.). I continued the backcrosses with the same pollen parent, up to the SB60 generation in 2013. Meiotic pairing was normal, forming 21 bivalents, in an SB56 plant.
Selfed and backcrossed seed fertilities of the (caudata)-Tve were observed in the entire backcross program. The backcrossed seed fertility (%) was highly variable, with the mean and S. E. of 68.10 ± 16.43. The linear regression to the backcrossed generation calculated using the records of the SB3 to SB60 generations to grasp the tendency of improvement or depression of the backcrossed seed fertility, Y, with progression of the backcross generations, X, turned out to be Y = 0.181X + 62.3 (%). The regression coefficient of 0.181 was non-significant at the 5 % level of probability, meaning the female fertility of (caudata)-Tve did not change consistently during 60 years of backcrossing. On the contrary, the selfed seed fertility was completely zero in most backcrossed generations. These results indicated that the caudata plasmon induced complete male sterility in Tve wheat, which did not show any sign of recovery by repeated backcrossing with the wheat pollen for 60 generations, proving persistence of the plasmon effect on male sterility induction.
Another prominent effect of the caudata plasmon on the wheat phenotype was production of germless grains, which was first noticed in the SB15 generation of (caudata)-Tve. Since then, occurrence of germless grains in (caudata)-Tve was examined in each backcross generation. Its frequency in every ten SB generations was 15.3 % (SB15-SB20), 14.7 % (-SB30), 28.5 % (-SB40), 14.2 % (-SB50) and 5.2 % (-SB60). Overall frequency of the germless grains was 11.8 % in (caudata)-Tve, as compared to 0.022 % in normal Tve, indicating more than 500 times increase in its frequency by the caudata plasmon. Genetic effect of the caudata plasmon to produce germless grains in Tve continued, at least, for 46 generations, from SB15 to SB60. These results demonstrated that the genetic effects of the caudata plasmon on male sterility induction and germless grain production to ‘Tve’ persistently expressed during 60 generations of backcrossing with the Tve pollen.
Reconstitution of Ae. caudata from Its Genome and Plasmon Separated for Half a Century and Paternal mtDNA Transmission in Wheat
Ae. caudata (genome, CC) was reconstructed from the genome of its native strain and the plasmon originated from it and coexisted with the genomes of Tve wheat (AABBDD) for 50 generations of repeated backcrosses (details of the procedure and the results will be published elsewhere). The reconstructed caudata plants resembled those of the caudata accession that provided the plasmon to (caudata)-Tve SB50 in general morphology and showed normal vigor and fertility. We analyzed 8 chloroplast and 5 mitochondrial simple sequence repeat (abbrev. SSR) loci out of 45 loci reported in wheat (Ishii et al. 2001, 2006) (Yotsumoto et al. unpubl.). The tentative results indicated that the reconstructed caudata had the same band patterns in most SSR loci, as those of the original caudata accession and (caudata)-Tve SB50. These results suggested that the caudata plasmon remained unchanged during coexistence with the wheat genomes for 50 generations.
Following the report of Laser et al. (1997) with triticale, Tsukamoto et al. (2000), Hattori et al. (2002), Kitagawa et al. (2002) and Kawaura et al. (2011) obtained evidence on the paternal transmission of mtDNA in alloplasmic wheats, which included coding regions of, at least, ten mitochondrial genes. Laser et al. (1997) and Kawaura et al. (2011) showed that the paternally transmitted mtDNAs were rarely transcribed in the heteroplasmic plants. This is compatible with the persistence of phenotypic effects of the caudata plasmon during the repeated backcrosses with wheat pollen. The constancy of mitochondrial SSR loci in the alloplasmon during the repeated backcrosses proved here, however, is hardly compatible with the paternal transmission of mtDNAs, because the majority of mtDNA molecules of an alloplasmic line at an advanced backcross generation is assumed to be the paternal (wheat) molecules, but this is not the case. Further studies are needed to clarify the cause of this discrepancy.
References
Hattori N, Kitagawa K, Takumi S, Nakamura C (2002) Mitochondrial DNA heteroplasmy in wheat, Aegilops and their nucleus-cytoplasm hybrids. Genetics 160:1619–1630
Ishii T, Mori N, Ogihara Y (2001) Evaluation of allelic diversity at chloroplast microsatellite loci among common wheat and its ancestral species. Theor Appl Genet 103:896–904
Ishii T, Takahashi C, Ikeda N et al (2006) Mitochondrial microsatellite variability in common wheat and its ancestral species. Genes Genet Syst 81:211–214
Kawaura K, Saeki A, Masumura T et al (2011) Heteroplasmy and expression of mitochondrial genes in alloplasmic and euplasmic wheat. Genes Genet Syst 86:249–255
Kihara H (1924) Cytologische und genetische Studien bei wichtigen Getreidearten mit besonderer Rücksicht auf das Verhalten der Chromosomen und die Sterilität in den Bastarden. Mem Coll Sci Kyoto Univ Series B 1:1–251
Kihara H (1930) Genomanalyse bei Triticum und Aegilops. Cytologia 1:263–284
Kihara H (1945) Genomanalyse bei Triticum und Aegilops. IX. Systematischer Aufbau der Gattung Aegilops auf genomanalytischer Grundlage. Cytologia 14:135–144
Kihara H (1951) Wheat – records of a biologist (in Japan). Chuokoron-sha, Tokyo, p 320
Kihara H (1959) Fertility and morphological variation in the substitution backcrosses of the hybrid Triticum vulgare x Aegilops caudata. Proc X Int Congr Genet 1:142–171
Kihara H (1982) Wheat studies – retrospect and prospects. Elsevier Sci. Publ. Co., Amsterdam, p 308
Kihara H, Lilienfeld F (1932) Genomanalyse bei Triticum und Aegilops. IV. Untersuchungen as Aegilops x Triticum- und Aegilops x Aegilops-Bastarden. Cytologia 3:384–456
Kihara H, Tanaka M (1970) Addendum to the classification of the genus Aegilops by means of genome-analysis. Wheat Inform Serv 30:1–2
Kihara H, Wakakuwa S (1935) Weitere Untersuchungen über die pentaploiden Triticum-Bastarde IV. Jpn J Botany 7:381–387
Kitagawa K, Takumi S, Nakamura C (2002) Evidence of paternal transmission of mitochondrial DNA in a nucleus-cytoplasm hybrid of timopheevi wheat. Genes Genet Syst 77:243–250
Laser B, Mohr S, Odenbach W, Oettler G, Kuck U (1997) Paternal and novel copies of the mitochondrial orf25 gene in the hybrid crop-plant triticale: predominant transcriptional expression of the maternal copy. Curr Genet 32:337–347
Lilienfeld AF (1951) H. Kihara: genome-analysis in Triticum and Aegilops. X. concluding review. Cytologia 16:101–123
Lilienfeld AF, Kihara H (1934) Genomanalyse bei Triticum und Aegilops. V. Triticum timopheevi Zhuk. Cytologia 6:87–122
Matsumura S (1936) Beziehungen zwischen Chromosomenzahlen und Sterilität sowie einigen morphologischen Eigenschaften in der F2-Generation des Bastardes T. polonicum x T. spelta. Jpn J Bot 8:189–204
Matsumura S (1940) Weitere Unterzuchungen über die pentaploiden Triticum-Bastarde. X. Kreuzungsversuche mit gemischten pollen. Jpn J Bot 10:477–487
Mukai Y, Maan SS, Panayotov I, Tsunewaki K (1978) Comparative studies of the nucleus-cytoplasm hybrids of wheat produced by three research groups. Proc IV Int Wheat Genet Symp 1:282–292
Ogihara Y, Tsunewaki K (1988) Diversity and evolution of chloroplast DNA in Triticum and Aegilops as revealed by restriction fragment analysis. Theor Appl Genet 76:321–332
Ohta S (1995) Detection of homoeologous chromosome pairing in interspecific diploid hybrids among wild wheat relatives. In: Tsunewaki K (ed) Plant genome and plastome – their structure and evolution. Kodansha, Tokyo, pp 103–114
Sakamura T (1918) Kurze Mitteilung uber die Chromosomenzahlen und die Verwandt-schaftsverhaltnisse der Triticum-Arten. Bot Mag (Tokyo) 32:150–153
Tsukamoto N, Asakura N, Hattori N et al (2000) Identification of paternal mitochondrial DNA sequences in the nucleus-cytoplasm hybrids of tetraploid and hexaploid wheat with D and D2 plasmons from Aegilops species. Curr Genet 38:208–217
Tsunewaki K (2009) Plasmon analysis in the Triticum-Aegilops complex – review. Breed Sci 59:455–470
Tsunewaki K, Wang GZ, Matsuoka Y (1996) Plasmon analysis of Triticum (wheat) and Aegilops. 1. Production of alloplasmic common wheats and their fertilities. Genes Genet Syst 71:293–311
Tsunewaki K, Wang GZ, Matsuoka Y (2002) Plasmon analysis of Triticum (wheat) and Aegilops. 2. Characterization and classification of 47 plasmons based on their effects on common wheat phenotypes. Genes Genet Syst 77:409–427
Wang GS, Matsuoka Y, Tsunewaki K (2000) Evolutionary features of chondriome divergence in Triticum (wheat) and Aegilops shown by RFLP analysis of mitochondrial DNAs. Theor Appl Genet 100:221–231
Winkler H (1920) Verbreitung und Ursache der Parthenogenese in Pflanzen- und Tierreiche. Fisher, Jena
Acknowledgments
I wish to thank Naoki Mori, Shigeo Takumi and Tatsuya Yotsumoto of the Kobe University, for their kind help and support for the present work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Tsunewaki, K. (2015). Prof. H. Kihara’s Genome Concept and Advancements in Wheat Cytogenetics in His School. In: Ogihara, Y., Takumi, S., Handa, H. (eds) Advances in Wheat Genetics: From Genome to Field. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55675-6_1
Download citation
DOI: https://doi.org/10.1007/978-4-431-55675-6_1
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55674-9
Online ISBN: 978-4-431-55675-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)