Abstract
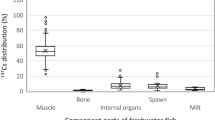
Ayu, Plecoglossus altivelis, is a herbivorous fish that is an important fishery resource and a key component of the food web in many Japanese streams. After the Fukushima Daiichi Nuclear Power Plant (FNPP) accident in March 2011, ayu were exposed to highly contaminated silt while feeding on benthic microalgae attached to riverbed stones. To understand the effects of radioactive contamination on ayu, radiocesium (134Cs + 137Cs) concentrations were analyzed in riverbed samples (microalgae and silt) and in the internal organs and muscle of ayu in five river systems in the Fukushima Prefecture between summer 2011 and autumn 2013. The concentrations of radiocesium in both the internal organs and the muscles of ayu declined over time. The radiocesium concentrations in the muscle were correlated with, but much lower than, those in the internal organs. The concentrations in the internal organs were correlated with those in the riverbed samples. The concentrations in the muscle were further correlated with ayu body size. Our results suggest that ayu ingest radiocesium while consuming silt and microalgae from the riverbed, and that a small proportion (about 15 %) is assimilated into the muscle of the fish.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
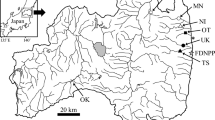
Ayu (Plecoglossus altivelis) is a herbivorous fish that is distributed throughout the Japanese Archipelago (Iguchi et al. 1999) (Fig. 17.1). The species exhibits an amphidromous and annual life cycle. After the winter juvenile stage in the sea, young ayu migrate into rivers and graze on benthic microalgae attached to the riverbed (Iguchi and Hino 1996). Ayu are also an important resource for humans and for avian species, such as the great cormorant Phalacrocorax carbo (Takahashi et al. 2006); therefore, the radionuclide contamination of ayu may have a significant effect on both humans and aquatic and terrestrial ecosystems. In Fukushima Prefecture, Iguchi et al. (2013) reported high levels of radionuclide contamination in the riverbed sediments. Ayu ingest silt while grazing on benthic microalgae, exposing themselves to the radiation from the contaminated sediments, including the silt component.
The aerosol-bound Cs was deposited on land and became integrated into the surface soil within 2 months after the Fukushima Daiichi Nuclear Power Plant (FNPP) disaster (Masson et al. 2011; Hirose 2012; Yasunari et al. 2011). Radionuclides subsequently spread over central and northern Honshu, Japan. The rivers in Honshu typically have steep gradients and are subject to erosion during snowmelt and typhoons (Yoshimura et al. 2005). As a result, contaminated soils were transported by the rivers from the mountains to the plains in Fukushima Prefecture (Evrard et al. 2013). To understand the route by which herbivorous fish are exposed to radiocesium, we measured radiocesium concentrations in riverbed samples (microalgae and silt) and in the internal organs and muscle of ayu in Fukushima Prefecture.
2 Relationship Between the Radiocesium Concentrations in Ayu Internal Organs and Muscle
Ayu (n = 166; fork length, 68–206 mm) were collected from five rivers in the Fukushima Prefecture by casting nets (periphery, 16 m; mesh size, 9 mm) between 9 July 2011 and 14 October 2013 (Fig. 17.2). The Niida and Kido Rivers were not sampled before May 2012 because of concerns about radiation safety in those areas. Collections of water, sediment, and riverbed samples, consisting primarily of benthic microalgae and silt, were made simultaneously at each site (Fig. 17.3) [see Tsuboi et al. (2015) for more details]. Radiocesium was detected in all 36 water and muddy sediment samples, 34 of 36 riverbed samples, and 119 internal organs and 98 muscle samples from 166 fish.
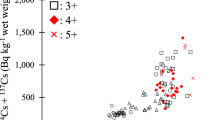
In 2013 the median 134Cs/137Cs ratio was 0.46 in all analyzed samples, which is identical to the value 2 years after the fallout from the FNPP. Radiocesium was detected in both the internal organs and the muscle of 84 individuals. Although there was a positive correlation between the concentrations of radiocesium in the internal organs and the muscle of ayu (r = 0.746, p = 0.006), the median concentration in the muscle was 14.5 % that of the median concentration in the internal organs (n = 84, p < 0.001). Thus, a small proportion (about 15 %) of the radiocesium ingested from the riverbed appears to be transferred to the muscle. Cesium strongly interacts with clay minerals, especially vermiculite and illite minerals (Comans and Hockley 1992). Furthermore, leaching experiments have demonstrated that radiocesium is relatively insoluble in the river suspended sediment in Fukushima, and that the adsorption of radiocesium to the suspended sediment was irreversible (Tanaka et al. 2013). Therefore, most of the radiocesium in the silt ingested by ayu is unlikely to be absorbed but will instead be excreted.
3 Biological and Environmental Factors Involved in the Temporal Pattern of Radiocesium Contamination
To evaluate temporal changes in 134Cs and 137Cs concentrations in water, muddy sediment, and ayu, we fitted a generalized linear mixed model (GLMM) with a Gaussian distribution of errors. The GLMM results suggest that the radiocesium concentrations in the muddy sediment but not river water have declined through time (river water: t = −1.016, p = 0.318; muddy sediment: t = −3.131, p = 0.004) (Figs. 17.4 and 17.5). The radiocesium concentrations have declined through time in both the internal organs and muscle of ayu (internal organs: t = −3.855, p < 0.001; muscle: t = −2.809, p = 0.006) (Fig. 17.6). The concentrations in the internal organs of ayu were positively correlated with those in the riverbed samples (i.e., fish prey) that were collected simultaneously with the ayu (t = 8.197, p < 0.001). In contrast, there was no correlation between the concentrations in the ayu muscle and the riverbed samples (t = −1.202, p = 0.261; Fig. 17.6). Thus, we conclude that herbivorous fish assimilate radiocesium from the microalgae and silt on the riverbed stones as they forage. Between 2011 and 2013, the activity concentration of radiocesium in the internal organs and the muscle of ayu declined, mainly because of the half-life of 134Cs (2.07 years), which is considerably shorter than the 30.1 years for 137Cs. However, the concentration of 137Cs in the whole ayu body tended to decrease during 2011 (Iguchi et al. 2013). Therefore, the decrease in the concentration of 137Cs in ayu cannot be explained only by the half-life of 134Cs. The activity concentration of 137Cs in the internal organs, which represented the majority of the 137Cs in ayu, was correlated with that in the riverbed samples. Therefore, the decrease of 137Cs in the riverbed, which may have been caused by flushing out of the contaminated soil from the mountains, would explain the decrease of 137Cs in ayu. In European lakes, 137Cs concentrations in fish muscle peaked a few years after the Chernobyl disaster (Jonsson et al. 1999; Smith et al. 2000). Then, the rate of decrease in muscle 137Cs concentrations was initially rapid, but later slowed. Conversely, in the rivers of Fukushima, the radiocesium contamination levels in ayu peaked immediately after the FNPP accident. The concentrations then decreased slowly, fluctuating with the transport of fresh polluted sediment from the mountains following snowmelt and typhoon events (Figs. 17.5 and 17.6).
Time-series of radiocesium concentrations in the river water. Symbols correspond to the collection sites in Fig. 17.2 (NI Niida River, KD Kido River, AK Abukuma River, SM Same River, OK Okawa River)
Time-series of radiocesium concentrations in muddy sediment. Symbols correspond to the collection sites in Fig. 17.2 (NI Niida River, KD Kido River, AK Abukuma River, SM Same River, OK Okawa River)
Time-series of radiocesium concentrations in the riverbed samples (i.e., fish dietary items; cross symbols) and the internal organs (i.e., stomach contents, stomach, gut contents, gut, liver, spleen, gonad; solid symbols) and the muscle (open symbols) samples from ayu collected in the five rivers (NI Niida River, KD Kido River, AK Abukuma River, SM Same River, OK Okawa River)
Ayu fork length was correlated not with concentrations of radiocesium in the internal organs but with that in the muscle (internal organs: t = −1.168, p = 0.246; muscle: t = 4.329, p < 0.001). The concentration of 137Cs in fish increases with fish size according to a power law relationship because of changes in prey items (Smith et al. 2002). For instance, the concentration in northern pike (Esox lucius) increased as the trophic level of prey increased from plankton to invertebrates and then to small fish. Indeed, the level of radiocesium contamination in fish at Fukushima increased according to the order herbivores (i.e., ayu) < omnivores < piscivores at Fukushima (Mizuno and Kubo 2013). A positive correlation was also observed for ayu body size relative to the muscle concentration of radiocesium. Thus, in this case, the positive correlation between ayu body size and radiocesium concentrations in their muscle could not be explained by a change in feeding patterns because the prey size and the prey items do not change as the ayu grows. Also, the time (season) of collection had no effect on the activity concentrations of radiocesium in ayu muscle. The activity concentration of radiocesium in fish is a function of uptake and elimination rates. In hatchery-reared ayu, assimilation efficiency decreases as the fish grow (Akutsu et al. 2001). Larger ayu therefore need much more food per unit weight gain than smaller individuals. Thus, at our study site, larger ayu have greater potential to accumulate radiocesium from microalgae on the riverbed stones than smaller ayu.
The overall radiocesium concentrations have declined with time in both the internal organs and muscle of ayu (Fig. 17.6). However, in some rivers surveyed, the radiocesium concentrations in the whole ayu body (i.e., internal organ and muscle) exceeded the Japanese standard limit for radiocesium in foods (100 Bq/kg-wet). Thus, fishing activities were banned in three of the five rivers during the sampling periods of this study (Niida, Kido, and Abukuma Rivers). Spatiotemporal monitoring of the levels of radiocesium in freshwater ecosystems, in areas close to human centers, should continue to increase our understanding of the long-term dynamics of radionuclide contamination and to reveal the effects on the biological and environmental characteristics of each ecosystem.
References
Akutsu M, Sawada M, Ishijima H (2001) A comparative testing among formula feeds on ayu. Bull Tochigi Pref Fish Exp Stat 44:3–4 (in Japanese)
Comans RNJ, Hockley DE (1992) Kinetics of cesium sorption on illite. Geochim Cosmochim Acta 56:1157–1164
Evrard O, Chartin C, Onda Y, Patin J, Lepage H, Lefèvre I, Ayrault S, Ottlé C, Bonté P (2013) Evolution of radioactive dose rates in fresh sediment deposits along coastal rivers draining Fukushima contamination plume. Sci Rep 3:3079
Hirose K (2012) Fukushima Dai-ichi nuclear power plant accident: summary of regional radioactive deposition monitoring results. J Environ Radioact 111:13–17
Iguchi K, Hino T (1996) Effect of competitor abundance on feeding territoriality in a grazing fish, the ayu Plecoglossus altivelis. Ecol Res 11:165–173
Iguchi K, Tanimura Y, Takeshima H, Nishida M (1999) Genetic variation and geographic population structure of amphidromous ayu Plecoglossus altivelis as examined by mitochondrial DNA sequencing. Fish Sci 65:63–67
Iguchi K, Fujimoto K, Kaeriyama H, Tomiya A, Enomoto M, Abe S, Ishida T (2013) Cesium-137 discharge into the freshwater fishery ground of grazing fish, ayu Plecoglossus altivelis, after the March 2011 Fukushima nuclear accident. Fish Sci 79:983–988
Jonsson B, Forseth T, Ugedal O (1999) Chernobyl radioactivity persists in fish. Nature (Lond) 400:417
Masson O, Baeza A, Bieringer J, Brudecki K, Bucci S, Cappai M, Carvalho FP, Connan O, Cosma C, Dalheimer A, Didier D, Depuydt G, De Geer LE, Vismes AD, Gini L, Groppi F, Gudnason K, Gurriaran R, Hainz D, Haldórsson O, Hammond D, Hanley O, Holeý K, Zs H, Ioannidou A, Isajenko K, Jankovic M, Katzlberger C, Kettunen M, Kierepko R, Kontro R, Kwakman PJM, Lecomte M, Leon Vintro L, Leppänen AP, Lind B, Lujaniene G, Ginnity PM, Mahon CM, Malá H, Manenti S, Manolopoulou M, Mattila A, Mauring A, Mietelski JW, Møller B, Nielsen SP, Nikolic J, Overwater RMW, Pálsson SE, Papastefanou C, Penev I, Pham MK, Povinec PP, Ramebäck H, Reis MC, Ringer W, Rodriguez A, Rulík P, Saey PRJ, Samsonov V, Schlosser C, Sgorbati G, Silobritiene BV, Söderström C, Sogni R, Solier L, Sonck M, Steinhauser G, Steinkopff T, Steinmann P, Stoulos S, Sýkora I, Todorovic D, Tooloutalaie N, Tositti L, Tschiersch J, Ugron A, Vagena E, Vargas A, Wershofen H, Zhukova O (2011) Tracking of airborne radionuclides from the damaged Fukushima Dai-ichi nuclear reactors by European networks. Environ Sci Technol 45:7670–7677
Mizuno T, Kubo H (2013) Overview of active cesium contamination of freshwater fish in Fukushima and eastern Japan. Sci Rep 3:1742
Smith JT, Comans RNJ, Beresford NA, Wright SM, Howard BJ, Camplin WC (2000) Chernobyl’s legacy in food and water. Nature (Lond) 405:141
Smith JT, Kudelsky AV, Ryabov IN, Daire SE, Boyer L, Blust RJ, Fernandez JA, Hadderingh RH, Voitsekhovitch OV (2002) Uptake and elimination of radiocaesium in fish and the “size effect”. J Environ Radioact 62:145–164
Takahashi T, Kameda K, Kawamura M, Nakajima T (2006) Food habits of great cormorant Phalacrocorax carbo hanedae at Lake Biwa, Japan, with special reference to ayu Plecoglossus altivelis. Fish Sci 72:477–484
Tanaka K, Sakaguchi A, Kanai Y, Tsuruta H, Shinohara A, Takahashi Y (2013) Heterogeneous distribution of radiocesium in aerosols, soil and particulate matters emitted by the Fukushima Daiichi Nuclear Power Plant accident: retention of micro-scale heterogeneity during the migration of radiocesium from the air into ground and river systems. J Radioanal Nucl Chem 295:1927–1937
Tsuboi J, Abe S, Fujimoto K, Kaeriyama H, Ambe D, Matsuda K, Enomoto M, Tomiya A, Morita T, Ono T, Yamamoto S, Iguchi K (2015) Exposure of a herbivorous fish to 134Cs and 137Cs from the riverbed following the Fukushima disaster. J Environ Radioact 141:32–37
Yasunari TJ, Stohl A, Hayano RS, Burkhart JF, Eckhardt S, Yasunari T (2011) Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc Natl Acad Sci U S A 108:19530–19534
Yoshimura C, Omura T, Furumai H, Tockner K (2005) Present state of rivers and streams in Japan. River Res Appl 21:93–112
Acknowledgements
This chapter was revised from a paper published by Tsuboi et al. (2015). The authors are grateful to Masato Murakami and Tomoko Okazaki for their assistance with the sample assays and data analyses. They also thank Kaoru Nakata and Kazuo Uchida for critical review of the manuscript. This study was supported by the Fisheries Agency, Ministry of Agriculture, Forestry and Fisheries, Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Tsuboi, Ji. et al. (2015). Spatiotemporal Monitoring of 134Cs and 137Cs in Ayu, Plecoglossus altivelis, a Microalgae-Grazing Fish, and in Their Freshwater Habitats in Fukushima. In: Nakata, K., Sugisaki, H. (eds) Impacts of the Fukushima Nuclear Accident on Fish and Fishing Grounds. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55537-7_17
Download citation
DOI: https://doi.org/10.1007/978-4-431-55537-7_17
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55536-0
Online ISBN: 978-4-431-55537-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)