Abstract
Their sizes and masses are the most fundamental properties of nuclei. They have simple mass number dependences which suggest that the nucleus behaves like a liquid and lead to the liquid-drop model for the nucleus. In this chapter we learn these bulk properties of nuclei and their applications to discussing nuclear stability, muon-catalysed fusion and the structure of heavy stars. As an example of the applications we discuss somewhat in detail the basic features of fission and nuclear reactors. We also mention deviations from what are expected from the liquid-drop model which suggest the pairing correlation and shell effects. We also discuss the velocity and the density distributions of nucleons inside a nucleus.
This is a preview of subscription content, log in via an institution.
Buying options
Tax calculation will be finalised at checkout
Purchases are for personal use only
Learn about institutional subscriptionsNotes
- 1.
It was 1911 when Rutherford submitted his article on the atomic model to a science journal. The idea and the formula of Rutherford were derived by stimulation of experimental results of his coworker Marsden who had been engaged in the study of scattering of alpha particles emitted from natural radioactive elements on matter. Furthermore, they have been confirmed experimentally to be correct by his collaborators Geiger and Marsden.
- 2.
The history of the progress and discoveries of modern physics in the period from late in the nineteenth century to the beginning of the twentieth century is vividly described in the book by E. Segré [1].
- 3.
R. Hofstadter was awarded the Nobel Prize in Physics 1961 for the study of high energy electron scattering with linear accelerator and the discovery of the structure of nucleon . He performed also systematic studies of nuclei by electron scattering.
- 4.
The electron scattering is a powerful method to learn the structure of nucleons mentioned in Chap. 1, and also to study nuclear excitations such as giant resonances and hypernuclei as well.
- 5.
The distribution of protons \(\rho _{\mathrm {p}}\) can be derived from \(\rho _C\) by taking into account the intrinsic structure of proton.
- 6.
The dips in diffraction pattern are buried by the distortion effects due to Coulomb force in the case of target nuclei with a large mass number such as Au. The diffraction pattern appears more clearly for the target nuclei with small mass number.
- 7.
In stable nuclei, the protons and neutrons distribute inside the nucleus almost in the same way. Here, we therefore treat the density distribution of protons and of nucleons as the same except for the absolute value. In these days, extensive studies are performed on the nuclei far from the \(\beta \)-stability line , which are called unstable nuclei . It is then getting known that some of them have very different distributions for protons and for neutrons. For example, the region where there exists only neutrons largely extends over the surface region in some nuclei such as \({}^{11}\) Li in the vicinity of the neutron drip line . Such layer is called the neutron halo . Recently, it is reported from the inelastic scattering of polarized protons (see [5]) and also from the elastic scattering of polarized electrons that even a typical stable nucleus \({}^{208}\) Pb has a larger radius of the neutron distribution than that of the proton distribution by 0.15–0.33 fm. Relatedly, the study of the existence of the region consisting of only neutrons, which is called neutron skin , is an active area of research.
- 8.
There exist deviations from the Woods–Saxon type for individual nucleus. They are explained by shell model .
- 9.
The value \(\rho _0\sim 0.17\,\mathrm {fm}^{-3}\) is widely accepted as the density of nuclear matter (see [6] for the argument about the detailed mass number dependence of the central density of nuclei with large mass number).
- 10.
Although the nucleus appears like a drop of liquid, it is not a classical liquid , but a drop of quantum liquid , since commutation relations govern the nucleus. In the classical liquid, the momentum space is not deformed even if it is spatially deformed. On the other hand, in the case of nuclei, a spatial deformation leads to the deformation in the momentum space through the uncertainty relation (see the footnote concerning quantum liquid in Sect. 8.3.4).
- 11.
Equations (2.24), (2.27) and (2.28) hold for stable nuclei. It has been found that the radii of unstable nuclei, especially of nuclei in the vicinity of neutron drip line , significantly deviate from these equations. For example, in the case of Li isotopes, the radius of \({}^{11}\) Li which has a neutron halo as mentioned before and thus called a halo nucleus or a neutron halo nucleus is much larger than what we expect from Eq. (2.27), although the radii of stable isotopes \({}^{6,7}\) Li and of the isotopes \({}^{8,9}\) Li which lie close to the stability line vary with A almost following Eq. (2.27).
- 12.
The measurement with high accuracy in the extreme forward region, where \(q^2\) is extremely small, is a difficult task and is a challenging subject in these days. In practice, one estimates the radius by analysing the data in the region where the measurement with high accuracy is doable, by assuming, e.g., the exponential or the Gaussian density distribution as shown in Fig. 2.5. Also, it is known that it is necessary to take into account the magnetic effects for the case of nucleons.
- 13.
As Fig. 2.5 shows, the experimental data for the region of small momentum transfer can be reproduced equally well by assuming the charge distribution in a proton to be either exponential or Gaussian type. Historically, the charge distribution of exponential type has been considered to be a good approximation, and correspondingly, the form factor of dipole type has been used. However, it is getting known from recent studies that the experimental data significantly deviate from the form factor of the dipole type, and it is argued that the charge distribution inside a proton is closer to a Gaussian type.
- 14.
The pair nuclei such as \({}^{15}_{~7}\text {N}_8\) and \({}^{15}_{~8}\text {O}_7\) which have interchanged numbers of protons and neutrons are called mirror nuclei to each other. Their energy levels are nearly equal, but shifted.
- 15.
One can take the opposite point of view that \(30\%\) is a large number. The relativistic descriptions of nuclear structure and nuclear reactions are also actively going on based on that point of view. The relativistic treatment has the advantage that the spin of nucleons is naturally introduced. In this book, we only touch the general framework of the relativistic mean-field theory in Sect. 6.3.
- 16.
The separation energy is also an important related physical quantity. It is the energy needed to remove a particle such as a nucleon or an alpha particle from the nucleus. For example, the separation energy of neutron is given by \(S_n(N,Z)\equiv B(A,Z)-B(A-1,Z)\) in terms of the binding energies of the relevant nuclei.
- 17.
The binding energy of a nucleus is usually calculated from the mass of the corresponding neutral atom \(M_{\mathrm {atom}}(A,Z)\) as
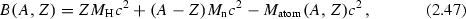
where \(M_{\mathrm {H}}\) is the mass of the Hydrogen atom.
- 18.
Exactly speaking, they have been obtained by using Eq. (2.47).
- 19.
We name deuteron and triton and denote by d and t in the case of nuclei, while name deuterium and tritium and denote by D and T in the case of neutral atoms.
- 20.
Those nuclei such as \({}^8_4\) Be, \({}_{~6}^{12}\) C , \({}^{16}_{~8}\) O whose atomic number and the mass number are the same multiples of those of \(\alpha \) particle are called \(\alpha \)-nuclei.
- 21.
The binding energy of \({}^8_4\)Be is less than twice that of \(\alpha \) by 0.1 MeV, so that \({}^8_4\)Be is unstable and exists only transiently.
- 22.
The nucleus which has the largest value of B / A is \({}^{62}_{28}\) Ni (see [14]).
- 23.
Only 5 nuclei, i.e., d, \({}^{6}_{3}\) Li , \({}^{10}_{~5}\) B , \({}^{14}_{~7}\) N , \({}^{180}_{~73}\) Ta (isomer), are stable among the so-called odd–odd nuclei , which have odd numbers for both the proton and the neutron numbers. Incidentally, it is \({}^{209}_{~83}\) Bi which has the largest atomic number among stable nuclei, although exactly speaking it decays with the half-life of \(1.9\times 10^{19}\) years. There exist no stable isotopes for Tc and Pm , whose atomic numbers are 43 and 61, respectively.
- 24.
It is related to the pairing correlation between nucleons, which will be discussed later in detail.
- 25.
It is the problem that the observed number of solar neutrinos is only about 1 / 2–1 / 3 of the theoretical prediction.
- 26.
In 1956, Alvarez et al. discovered a trajectory of muon which stops in the liquid hydrogen contaminated with deuterium. The flight length in matter is determined by the stopping power . The length 1.7 cm of the trajectory observed by Alvarez et al. exactly matched the flight length of muon with energy of 5.4 MeV which is released in the p + d \(\rightarrow {}^3\)He reaction.
- 27.
The threshold energies for \(\alpha \)-decay, and those for emitting several \(\alpha \) particles, and also the threshold energy of the decay of \({}^{24}\) Mg into two \({}^{12}\)C nuclei, taken from [19].
- 28.
The synthesis of \({}^{12}\)C in stars proceeds as \({}^{8}\)Be + \(\alpha \rightarrow {}^{12}\)C(\(0^+_2\)), \({}^{12}\)C\((0^+_2) \rightarrow {}^{12}\)C\((0^+_{\mathrm {g.s.}})\) In that sense, the \(0^+_2\) state of \({}^{12}\)C plays a crucial role in the synthesis of heavy elements, and is named Hoyle-state after F. Hoyle who predicted the existence of this state.
- 29.
In the case of \({}^{12}\)C, the spin–orbit interaction reduces the extent of deformation and increases the excitation energy of the \(2^+_1\) state. From the point of view of shell model, a force with smaller spin–orbit splitting leads to a high degeneracy and to a larger deformation due to the Jahn–Teller effect, while a force with a strong spin–orbit force leads to a large splitting between the \(p_{3/2}\) and \(p_{1/2}\) states. Consequently, the degeneracy disappears and the spherical shape is predicted to appear. Inversely, if one considers from the point of view of the cluster model, too large deformation or clusterization is predicted and consequently the excitation energy of the \(2^+_1\) state becomes too low compared with the experimental data if one assumes the cluster state which extends the pure state of the maximum spatial symmetry of the shell model denoted by \((0s)^4(0p)^8 [444]^{11}L\) configuration by introducing a finite distance between three alpha clusters, but ignores the spin–orbit interaction, and the experimental data can be well reproduced by admixing states with lower spatial symmetry by the spin–orbit interaction.
- 30.
In the case when the electronic states degenerate in energy in a polyatomic molecule where the atoms have a highly symmetric geometrical configuration such as a regular polyhedron, it becomes energetically more stable if the degeneracy is removed by assuming a configuration of atoms with lower symmetry. This is known as the Jahn–Teller effect. Similar situations occur in many physical systems including metal microclusters.
- 31.
A statement often appears in the literature that the central part of massive stars consists of \({}^{56}_{26}\) Fe due to the fact that the nucleus which has the largest binding energy per nucleon is \({}^{56}_{26}\)Fe. However, in reality, the nucleus which has the maximum binding energy per nucleon is \({}^{62}_{28}\)Ni. It is understood that the reason why the major component of the core is \({}^{56}_{26}\)Fe despite this fact is because \({}^{62}_{28}\)Ni is hard to be synthesized by nuclear reactions in stars, while \({}^{56}_{26}\)Fe can be easily synthesized as the last nucleus in the Si-burning reaction.
- 32.
The temperature of the central part has to be sufficiently high in order for the core of Fe and Ni to be formed in the central part of stars. In the stars whose mass is not so large, the central part therefore consists of nuclei with smaller mass number than that of Fe, though they have an onion layer structure. For example, the central part of stars whose mass lies between \(8M_{\odot }< M < 12M_\odot \) consists of O, Ne and Mg.
- 33.
There is an alternative way to classify the supernova explosion into the type II and I, respectively, depending on whether the spectrum contains the hydrogen line or not.
- 34.
At present, there exist several refined mass formulae [24] reflecting various studies such as those of unstable nuclei , superheavy elements and nucleosynthesis of elements through r-process. In those refined mass formulae, for example, the effects of surface diffuseness and of the symmetry energy correction to the volume as well as the surface terms are considered.
- 35.
If we define the nuclear surface tension \(\sigma \) by \(\sigma \equiv b_{\mathrm {surf}}A^{2/3}/4\pi (r_0A^{1/3})^2\), then \(\sigma \sim 1\,\text {MeV/fm}^2\).
- 36.
\({}^{150}_{~58}\) Ce and \({}^{208}_{~80}\) Hg are unstable nuclei. The relationship \(N\sim 1.6Z\) should be taken only as a rough relationship that holds in the region of large mass number. To be accurate, it is safer to use Eq. (2.59).
- 37.
In the spontaneous fission or in the induced fission by a small energy, hence with low excitation energy, fission with a striking mass-asymmetry occurs as shown in Fig. 2.17 because of the shell effect which we study in Chap. 5. Also, a few neutrons are emitted simultaneously in each spontaneous fission and in each thermal neutron-induced fission. For example, the average numbers of neutrons emitted in thermal neutron-induced fission of \({}^{235}\) U and \({}^{239}_{~94}\) Pu are 2.4 and 2.9, respectively [27].
- 38.
Hahn and Strassmann, who were studying the decay of U by irradiation with neutrons, discovered fission by identifying the fission fragments, which had been believed to be \({}_{88}\)Ra, \({}_{89}\)Ac, \({}_{90}\)Th, as \({}_{56}\)Ba, \({}_{57}\)La, \({}_{58}\)Ce. Lise Meitner made also a significant contribution to the series of studies as a collaborator of Hahn.
- 39.
The basic data and theoretical descriptions of fission are given in detail in [27].
- 40.
As we learn in Chap. 7 many nuclei which undergo spontaneous fission such as actinides to which U belongs are deformed even in their ground states due to the shell effect, i.e., a quantum effect, and the long axis is about 30\(\%\) longer than the short axis. Also, as shown in Fig. 2.17, most of the fission is an asymmetric fission, where the masses of the two fission fragments are significantly different. Following the original picture of Bohr–Wheeler which is based on the liquid-drop model, we develop in this book our discussions by assuming that the ground state of nuclei prior to fission, which is a metastable state, is spherical. Incidentally, the shell effect was discovered and the shell model has been established much later.
- 41.
The heat associated with a nuclear reaction or with an atomic or a molecular reaction is called the Q-value of the reaction. It becomes positive or negative for exothermic or endothermic reactions, respectively. It corresponds to the energy released by fission in the case of a nuclear fission.
- 42.
Fission is stimulated if the nucleus is irradiated by a photon, or neutron or by other particles. Such reaction is called induced fission and is distinguished from the spontaneous fission. It is represented as, for example, (\(\gamma \),f) and (n,f) for photofission and neutron-induced fission, respectively.
- 43.
About 200 MeV is a measure of the Q-value for the fission of actinides such as U.
- 44.
In reality, fission is a quantum tunneling in a multidimensional space with many degrees of freedom.
- 45.
In the case of \(\alpha \)-decay, the potential barrier to be transmitted by a quantum tunneling is located outside the sum of the radii of the \(\alpha \) particle and the daughter nucleus. In other words, if we consider the \(\alpha \)-decay as a kind of fission process, then the potential barrier appears outside the scission point. That is the reason why the coordinate for the \(\alpha \)-decay can be taken to be the coordinate of the relative motion.
- 46.
We do not consider \(\lambda =1\), because it describes the translational motion of the nucleus as a whole. There holds the relationship \(\alpha _\lambda =\sqrt{(2\lambda +1)/4\pi }\alpha _{20}\) between the \(\alpha _\lambda \) in this section and the deformation parameters \(\alpha _{\lambda \mu }\), which will be introduced in Chap. 7.
- 47.
The critical nucleus is \((A_{\mathrm {cr}},\,Z_{\mathrm {cr}})\sim (340,131)\) if we use \(N\sim 1.6Z\). However, in more refined estimates which go beyond the quadrupole deformation we used here the potential barrier by the liquid-drop model almost disappears when the atomic number exceeds the critical value \(Z_{\mathrm {cr}}\sim 100\) [30].
- 48.
Bohr and Wheeler estimated the value of \(E_f\) for the other nuclei by assuming \((Z^2/A)_{\mathrm {cr}}\) to be 47.8 from the experimental data that \(E_f\) is about 6 MeV for \({}^{239}\)U.
- 49.
The scattering cross section plotted as a function of the collision energy is called the excitation function.
- 50.
In classical mechanics, a particle is reflected at the position where the total energy of the particle becomes identical with the potential energy. Such positions are therefore called classical turning points or simply turning points.
- 51.
The reactor must have become fairly different from the present one if there existed no pairing correlation between nucleons.
- 52.
The information on both the potential surface and the effective mass is needed in order to theoretically evaluate the fission width. Nowadays, the potential surface can be obtained with relatively high accuracy using, for example, the macroscopic–microscopic method , which refines the liquid-drop model by adding the shell correction and will be described in Sect. 7.6.1. On the contrary, it is difficult to estimate the mass parameter with high reliability. One can hope to obtain important phenomenological information on the effective mass for fission by using the results of this exercise.
- 53.
- 54.
It is Fermi and his collaborators who succeeded in the chain reaction of the induced fission of U by a slow neutron for the first time, and the experiment was performed at the University of Chicago on December 2, 1942.
- 55.
In commercial reactors, the enrichment of \({}^{235}\)U is typically about 3–4%.
- 56.
The center of mass energy spectrum of neutrons emitted from a nucleus in the excited state with temperature T is expected to be given by \(\varepsilon _{\mathrm {n}} \exp (-\varepsilon _{\mathrm {n}}/T)\), and is well described by the theory called the evaporation theory . The dashed line in Fig. 2.30 represents the energy spectrum expected from the evaporation theory transformed to that in the laboratory frame by taking into account the finite velocity of the fission fragment.
- 57.
The reactor which uses high energy neutrons, instead of neutrons which have been cooled down to thermal neutrons due to the elastic scattering in the neutron moderator, is called fast reactor or fast neutron reactor.
- 58.
One of the powerful methods to obtain the potential surface with multiple-potential barriers is the macroscopic–microscopic method which modifies the liquid-drop model by adding shell correction (see Sect. 7.6.1). In the fission of \({}^{231}\) Th , the first fission barrier is lowered by axial asymmetry. The axial symmetry is recovered in the second fission barrier. However, the barrier height gets lower by considering mass asymmetry, and furthermore there appears the third potential minimum.
- 59.
The ratio of the length of the long axis to that of the short axis is 2:1 for the second potential minimum.
- 60.
The ratio of the length of the long axis to that of the short axis is about 1.3 for usual deformed nuclei such as the ground state of U. In comparison, the moment of inertia of fission isomers is 2.2 times that of the ground state for \({}^{238}\)U as shown in Fig. 2.34. \({}^8\)Be, which has the dumb-bell structure of two touching \(\alpha \) particles, has also 2:1 for the ratio of the length of the long axis to that of the short axis, and hence is one of the hyperdeformed states.
References
E. Segré, From X-Rays to Quarks: Modern Physicists and Their Discoveries (W. H. Freeman and Company, San Francisco, 1980)
M. Nogami, Nuclear Physics, Japanese edn. (Shoukabou, Tokyo, 1973)
T. de Forest Jr., J.D. Walecka, Adv. Phys. 15, 1 (1966)
B. Hahn, D.G. Ravenhall, R. Hofstadter, Phys. Rev. 101, 1131 (1956)
A. Tamii et al., Phys. Rev. Lett. 107, 062502 (2011)
W.N. Cottingham, D.A. Greenwood, An Introduction to Nuclear Physics, 2nd edn. (Cambridge University Press, Cambridge, 2001)
R. Hofstadter, F. Bumiller, M.R. Yearian, Rev. Mod. Phys. 30, 482 (1958)
N. Takigawa, M. Ueda, M. Kuratani, H. Sagawa, Phys. Lett. B 288, 244 (1992)
J.S. Al-Khalili, J.A. Tostevin, Phys. Rev. Lett. 76, 3903 (1996)
B. Abu-Ibrahim, Y. Suzuki, Phys. Rev. C 61, 051601 (2000)
J. Kasagi, H. Yuki, T. Baba, T. Noda, T. Ohtsuki, A.G. Lipson, J. Phys. Soc. Jpn. 71, 2881 (2002)
Y. Kato, N. Takigawa, Phys. Rev. C 76, 014615 (2007). And references therein
G. Audi, A.H. Wapstra, C. Thibault, Nucl. Phys. A 729, 337 (2003)
R. Shurtleff, E. Derringh, Am. J. Phys. 57, 552 (1989)
T. Arima, Master thesis, Tohoku University
W.-M. Yao et al. (Particle Data Group), J. Phys. G 33, 1 (2006)
L. Alvarez et al., Phys. Rev. 105, 1127 (1957)
S.E. Jones, Nature 321, 127 (1986)
K. Ikeda, N. Takigawa, H. Horiuchi, Prog. Theor. Phys. Suppl. E68, 464 (1968)
K. Ikeda, T. Marumori, R. Tamagaki, H. Tanaka, J. Hiura, H. Horiuchi, Y. Suzuki, F. Nemoto, H. Bando, Y. Abe, N. Takigawa, M. Kamimura, K. Takada, Y. Akaishi, S. Nagata, Prog. Theor. Phys. Suppl. 52 (1972); K. Ikeda, R. Tamagaki, S. Saito, H. Horiuchi, A. Tohsaki-Suzuki, M. Kamimura, Prog. Theor. Phys. Suppl. 62 (1977)
H. Horiuchi, K. Ikeda, K. Kato, Prog. Theor. Phys. Suppl. 192, 1 (2012)
C.M. Lederer, V. Shirley, Table of Isotopes, 7th edn. (Wiley, New York, 1978)
N. Takigawa, A. Arima, Nucl. Phys. A 168, 593 (1971)
P. Möller, J.R. Nix, W.D. Myers, W.J. Swiatecki, At Data Nucl. Tables 59, 185 (1995); P. Möller, J.R. Nix, Nucl. Phys. A 536, 20 (1992); H. Koura, M. Uno, T. Tachibana, M. Yamada, Nucl. Phys. A 674, 47 (2000); H. Koura, T. Tachibana, M. Uno, M. Yamada. Prog. Theor. Phys. 113, 305 (2005)
S. Yusa, private communication
K.F. Flynn, L.E. Glendenin, ANL-7749 (1970); cited in [27]
R. Vandenbosch, J.R. Huizenga, Nuclear Fission (Academic Press, New York, 1973)
O. Hahn, F. Strassmann, Naturwiss. 27, 11 (1939)
N. Bohr, J.A. Wheeler, Phys. Rev. 56, 426 (1939)
F. Ivanyuk, K. Pomorski, Phys. Rev. C 79, 054327 (2009); Int. J. Mod. Phys. E 19, 514 (2010)
A.H. Wapstra, G. Audi, Nucl. Phys. A 432, 1 (1985)
W.J. Swiatecki, Phys. Rev. 100, 937 (1955)
K. Yagi, Nuclear Physics, Japanese edn. (Asakura, Tokyo, 1971)
W.S.C. Williams, Nuclear and Particle Physics (Clarendon press, Oxford, 1991)
G.D. James, J.E. Lynn, L.G. Earwaker, Nucl. Phys. A 189, 225 (1972)
J. Blons, Nucl. Phys. A 502, 121c (1989)
P. Möller, A.J. Sierk, T. Ichikawa, A. Iwamoto, R. Bengtsson, H. Uhrenholt, S. Åberg, Phys. Rev. C 79, 064304 (2009)
P. Möller, J.R. Nix, in Proceedings of the Third IAEA Symposium on the Physics and Chemistry of Fission, Rochester, 1973, Vol. I (International Atomic Energy Agency, Vienna, 1974), p. 103
R. Vandenbosch, Nucl. Phys. A 502, 1c (1989)
V. Metag, D. Habs, H.J. Specht, Phys. Rep. 65, 1 (1980); The original paper is H.J. Specht, J. Weber, E. Konecny, D. Heunemann. Phys. Lett. B 41, 43 (1972)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2017 Springer Japan
About this chapter
Cite this chapter
Takigawa, N., Washiyama, K. (2017). Bulk Properties of Nuclei. In: Fundamentals of Nuclear Physics. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55378-6_2
Download citation
DOI: https://doi.org/10.1007/978-4-431-55378-6_2
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55377-9
Online ISBN: 978-4-431-55378-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)