Abstract
The separation conditions for iodine species were investigated to analyze 129I in contaminated water and tree samples generated from the Fukushima Daiichi Nuclear Power Station (FDNPS). Inorganic iodine species in the samples from FDNPS were thought to be iodide (I−) and iodate (IO3 −); therefore, the behaviors of these species during separation using a solid-phase extraction sorbent, Anion-SR, for water samples and combustion for tree sample were studied. When the amount of I was 1 μg and used within a few hours, I− was extracted with the Anion-SR in 3 M NaOH and diluted HCl (pH 2) solutions, whereas IO3 − was only slightly extracted in these solutions. In contrast, 15 ng I− with a larger amount of IO3 − (1 μg I) in the diluted HCl (pH 2) and allowed to stand for 1 day was only slightly recovered. It is possibly that I− was changed to another species in a day in this condition. Iodate was successfully reduced to I− with NaHSO3 in the diluted HCl solution and extracted with the Anion-SR. Consequently, the solution condition to analyze both I− and IO3 − using Anion-SR was observed to be the diluted HCl at pH 2 with a reductant. For the tree samples, a combustion method was applied and the rate of temperature increase was optimized to avoid anomalous combustion. Greater than 90 % recovery was obtained for both I− and IO3 −, and the chemical species in the trap solutions was observed to contain I−.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- 129I
- Combustion
- Fukushima Daiichi Nuclear Power Station
- Iodine species
- Isotopic exchange
- Solid-phase extraction
1 Introduction
Because of the accident, a large amount of radioactive waste was generated at the Fukushima Daiichi Nuclear Power Plants (FDNPP). To establish the waste management strategy, the radioactivity inventory has to be evaluated. Iodine-129 is one of the important nuclides of which the radioactivity has to be evaluated. Although I− is considered a major species of 129I generated in the reactor, IO3 − and I2 are possibly generated, depending on the reactor conditions [1]. Furthermore, because seawater was introduced to the reactors for cooling down in the early phase of the accident and seawater contains the natural iodine species, 127IO3 −, an isotope exchange reaction between 127IO3 − and 129I− may have occurred. Therefore, analytical conditions to determine total I content, in this case IO3 − and I−, in water and tree samples were investigated in the current work.
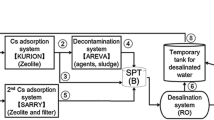
Presently, contaminated water is accumulating in the basement of the reactor and turbine buildings at FDNPP. The accumulated water-processing equipment was installed to decontaminate and to desalinate. Consequently, secondary waste such as spent zeolite and sludge is generated. To evaluate the radioactivity inventory of the waste indirectly, water samples were collected from the inflow and outflow of the apparatus [2]. The contaminated water contains high levels of radioactivity of 137Cs, 90Sr, and other radionuclides. To limit radiation exposure of the analyst, rapid chemical separation of iodine species from these radionuclides is required. Chemical separation studies using the solid-phase extraction sorbent Anion-SR have been reported to rapidly separate I− from major fission products such as Cs and Sr in contaminated water samples [3]. However, Anion-SR essentially extracts only I− and not IO3 −. Therefore, reduction of IO3 − to I− is required to analyze total I. In this study, NaHSO3 was used as the reductant and the solution conditions were studied to reduce IO3 − to I−.
Because of the hydrogen explosion of FDNPP, trees on the site were contaminated by the radionuclides. Many of the trees were cut down to provide space to install tanks storing the contaminated water. Consequently, approximately 40,000 m3 of trees were stored in the site as radioactive waste [4]. A combustion method was used to analyze 129I in cement, ash, and soil samples [5, 6]. To apply a combustion method for the tree samples, there were some subjects: evaporation and deposition of the organic materials and anomalous combustion. Therefore, decomposition of organic material to CO2 and H2O using oxidant was examined. In addition, the rate of temperature increase was controlled to avoid anomalous combustion. Furthermore, the influence of the chemical species, IO3 − or I−, on recovery was studied.
2 Experimental
2.1 Reagents
Ultrapure grade NaOH solution (3 M) was purchased from Kanto Kagaku. Ultrapure grade of tetramethyl ammonium hydroxide (TMAH) solution was purchased from Tama Chemicals. The other reagents were all analytical grade or higher and purchased from Wako Chemical. Empore solid extraction disks, Anion-SR, were purchased from 3 M. Standard solution of 129I (35.8 kBq/g 129I; chemical composition, 50 μg/g NaI and 50 μg/g Na2S2O3 in H2O) was purchased from AREVA and diluted with H2O before use. Potassium iodide (analytical grade, 99.9 %) and KIO3 (analytical standard material grade, 99.98 %) were purchased from Wako Pure Chemical Industries, and 127I− and 127IO3 − were prepared from these reagents, respectively, because the stable iodine isotope is only 127I.
2.2 Separation Using Anion-SR
Figure 27.1 shows the experimental procedure for the solution samples. Sodium iodide-129 (129I: 0.1 Bq = 15 ng) and K127IO3 − (127I: 1 μg) were added to 50 ml 3 M NaOH solution or 50 ml diluted HCl solution (pH = 2) with and without reductant (0.1 ml 0.1 M NaHSO3) to study iodine species behavior in the analysis using Anion-SR. After addition of the iodine species, the solutions were allowed to stand for 1 day before separation with Anion-SR. The reductant was added approximately 20 min before the separation. The operation of Anion-SR was based on Shimada et al. [3]. Briefly, the Anion SR disk was centered on the base of the filtration funnel and the reservoir was clamped on the top of the disk. The appropriate solution was poured into the reservoir followed by suction filtration The Anion-SR disk was conditioned with acetone, methanol, ultrapure water, 4 w/v% NaOH, and ultrapure water. After conditioning, the sample solution was introduced into the Anion-SR disk and washed with ultrapure water. The extracted I was recovered with 9.5 ml 1 M HNO3. To oxidize I− to IO3 −, 0.1 ml NaClO solution (effective Cl concentration, >5 %) was added to the recovered solution. Additionally, 0.1 ml 2 ppm Rh standard solution was added to the recovered solution as an internal standard. Finally, 1 M HNO3 was added to the recovered solution to a final volume of 10 ml. The concentration of I was measured by inductively coupled plasma mass spectrometry with dynamic reaction cell (DRC-ICP-MS). In the reaction cell, oxygen gas was collided with ions. Because the order of ionization potential is I > O > Xe, O reacts with Xe to neutralize but I does not react with O. As a result, the count of 129Xe, impurity of Ar gas, was decreased. The experimental conditions of DRC-ICP-MS were consistent with the conditions reported by Kameo et al. [7]. Percent recovery was calculated as in Eq. (27.1).
The separation experiment to determine percent recovery was carried out twice, and the uncertainty was quantified by the dispersion in these two measurements.
3 Combustion Method
A known amount of I− or IO3 − was added to 1 g pine bark, representative of tree samples taken at the establishment of the Japan Atomic Energy Agency, and the bark was put in a wet oxygen gas line set in an electric furnace. Because smoking was observed with noncontrolled temperature increase, especially around 240 °C, the rate of temperature increase in the range from 100 °C to 300 °C was controlled by steps and slow. The vaporized I was trapped in three steps of 20 ml 2 % TMAH solution. Because insoluble organic material was deposited in the gas line and the trap, an oxidant (8.2 g hopcalite II) was set between the sample and traps to decompose it (Fig. 27.2). The temperature of the oxidant was set to 500 °C before the temperature increase of the pine bark sample. The temperature of the sample was kept at 500 °C for 1 h and then increased to 900 °C for 1 h to vaporize iodine species in the sample. The extracted I in the traps was measured by DRC-ICP-MS.
4 Results and Discussion
4.1 Separation Using Anion-SR
Good recovery of I− (as 129I−) from the Anion-SR was observed in the 3 M NaOH solution, but only minor amounts of IO3 − (as 127IO3 −) were recovered (Table 27.1). The percent recoveries did not appear to be dependent on the reductant because good recoveries of I− and poor recoveries of IO3 − were observed regardless of the presence or absence of NaHSO3. This result supports the expectation that 129I− is extracted and 127IO3 − is not extracted by the Anion-SR, as 127IO3 − was not reduced to 127I− by NaHSO3 in 3 M NaOH solution, and the isotopic exchange reaction between 127IO3 − and 129I− and reduction from 127IO3 − to 127I− were negligible at least for 1 day. It is reported that 129I in seawater offshore of Fukushima is mainly 129I− regardless of the presence of large amounts of natural 127IO3 − [8].
In the diluted HCl solution at pH 2, 129I and 127I were recovered in the presence of the reductant and were not recovered without the reductant (Table 27.1). Both the isotopes behaved similarly in this case despite the difference in the initial chemical species, 129I− and 127IO3 −. In contrast, the experiments using 1 μg 127I− in the diluted HCl solution at pH 2 without the reductant and standing, higher recovery of 127I−, 72 ± 6 %, was obtained. Although the influence of the I− amount on recovery cannot be wholly denied, it is possible that a considerable amount of 129I− would be changed to another chemical species in the diluted HCl solution in a day. In addition, the changed chemical species and IO3 − were reduced to I− by the reductant in this experimental condition. Consequently, inorganic iodine species were analyzed at pH 2 with the reductant, and only I− was analyzed in 3 M NaOH. It is possibly to apply speciation methods to analyze I− and IO3 −, depending on the solution conditions.
4.2 Combustion Method
When 1 g pine bark was combusted by the electric furnace without control, smoking was observed at the temperature range from 200 ° to 300 °C, especially at 240 °C. Therefore, a stepwise and slow rate of temperature increase was introduced. When smoking was observed, the gap of the setting temperature was decreased and holding time at the temperature was prolonged. Finally, smoking was not observed by the controlled rising temperature program shown in Fig. 27.3, in which the increments of 50 °C for 20 min from 100 °C to 200 °C, and in increments of 15 °C for 20 min from 200 °C to 300 °C.
Tetramethyl ammonium hydroxide solutions containing known amounts of I− or IO3 − were prepared to make calibration curves for the DRC-ICP-MS measurement. However, the counts of mass number 127 for IO3 − were essentially at background levels, which indicated that measurement of IO3 − in 2 % TMAH solution was not available in our instrument. It is considered that I is vaporized as I2 by the combustion and I2 is trapped in an alkaline solution as I−. Therefore, the calibration curve was prepared from I− solutions.
Combustion experiments of pure pine bark sample and pine bark samples spiked with 50 μg I as I− or IO3 − were performed and the amounts of 127I in the traps were determined by DRC-ICP-MS with the calibration curve previously mentioned (Table 27.2). Greater than 90 % recovery was obtained for both I− and IO3 − spiked samples in the first trap. This result suggested that the chemical species of I in the traps was I− regardless of the initial chemical species.
5 Conclusion
To analyze 129I in the contaminated water from FDNPS, separation of I from radionuclides such as 137Cs and 90Sr using Anion-SR and measurement by DRC-ICP-MS are convenient and effective. As a solution condition, a diluted HCl solution of pH 2 with reductant was required to analyze inorganic iodine species using Anion-SR.
To analyze 129I in tree samples from FDNPS, a combustion method is suitable. Both I− and IO3 − were vaporized by combustion and trapped in the 2 % TMAH solution as I− with high recovery (>89 %). Anomalous combustion was avoided by the stepwise and slow temperature increase in the range from 100 ° to 300 °C.
References
Tigeras A, Bachet M, Catalette H, Simoni E (2011) Prog Nucl Energy 53:504–515
Tanaka K, Yasuda M, Watanabe K, Hoshi A, Higuchi H, Kameo Y (2013) Proceedings of the 14th workshop on environmental radioactivity, Tsukuba, pp 17–26
Shimada A, Sakatani K, Kameo Y, Takahashi K. J Radioanal Nucl Chem. Accepted for publication
Tanaka K, Shimada A, Hoshi A, Yasuda M, Ozawa M, Kameo Y (2014) J Nucl Sci Technol 51(7–8):1032–1043
Ishimori K, Haraga T, Shimada A, Kameo Y, Takahashi K (2010) Ibaraki (Japan): Japan Atomic Energy Agency; JAEA-technology 2010–016 (Japanese)
Toyama C, Muramatsu Y, Uchida Y, Igarashi Y, Aoyama M, Matsuzaki H (2012) J Environ Radioact 113:0116–0122
Kameo Y, Ishimori K, Shimada A, Takahashi K (2012) Bunseki Kagaku 61(10):845–849
Hou X, Povinec PP, Zhang L, Shi K, Biddulph D, Chang C-C, Fan Y, Golser R, Hou Y, Jeskovsky M, Tim Jull AJ, Liu Q, Luo M, Steier P, Zhou W (2013) Environ Sci Technol 47:3091–3098
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Shimada, A. et al. (2015). Development of a Rapid Analytical Method for 129I in the Contaminated Water and Tree Samples at the Fukushima Daiichi Nuclear Power Station. In: Nakajima, K. (eds) Nuclear Back-end and Transmutation Technology for Waste Disposal. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55111-9_27
Download citation
DOI: https://doi.org/10.1007/978-4-431-55111-9_27
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55110-2
Online ISBN: 978-4-431-55111-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)