Abstract
Spermatozoa must decode environmental and cellular cues to succeed in fertilization, and this process relies heavily on ion channels. New observations bring to light the relevant participation of Cl− channels and anion transporters in some of the main sperm functions. Here we review the evidence that indicates the participation of Cl− channels in motility, maturation, and the acrosome reaction (AR), and what is known about their molecular identity and regulation. Our better understanding of sperm anion transport will yield tools to handle some infertility problems, improve animal breeding and preserve biodiversity, and develop selective and secure male contraceptives.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Spermatozoa must find and fertilize the egg to deliver their genetic information and generate a unique individual in organisms of sexual reproduction. Ion pumps and transporters are used by cells to build up and maintain ion concentration gradients across their membranes. These ionic gradients allow cells to respond to their changing environment, to signals from other cells and to perform secondary transport. Ion channels can transport millions of ions per second, which enables them to rapidly modify the cell electric potential and the concentrations of internal second messengers within a wide time range, depending on their mode of regulation (Hille 2001).
Spermatozoa are small, differentiated, and morphologically complex cells (Yanagimachi 1994). They must be endowed with decoding systems for multiple signals along their journey to reach the egg to succeed in fertilizing it. During their maturational convoluted journey through the epididymis and the female reproductive tract, mammalian sperm encounter many environmental changes (Dacheux et al. 2012; Hung and Suarez 2010; Visconti et al. 2011). Although significant advances have been made in recent years, the set of ion channels and transporters needed for sperm to achieve fertilization is still not fully known (Darszon et al. 2011; Lishko et al. 2012; Publicover and Barratt 2012). Ion channel inhibition and knockout experiments have clearly revealed the major role these transporters play in sperm maturation, the regulation of motility, and the acrosome reaction (AR) (Darszon et al. 2011; Lishko et al. 2012). As gene transcription and protein synthesis seem not to occur in mature sperm, their proteins are generated during spermatogenesis (Baker 2011).
Considering that ion channels are minor membrane protein components, demonstrating the functional presence of a particular ion channel in spermatozoa needs controlled immunological or proteomic detection combined with electrophysiological, ion-sensitive fluorescent functional assays and pharmacology. When possible, eliminating the specific ion channel from spermatozoa might unravel its function (Kirichok et al. 2006; Santi et al. 2010). Initial glimpses of the properties of some sperm ion channels were derived from planar bilayers with incorporated sperm plasma membranes (reviewed by Darszon et al. 1999). Notably, some of the first sperm single-channel recordings were of K+ and Cl− channels obtained in planar bilayers with incorporated sea urchin sperm plasma membranes (Labarca et al. 1996; Lievano et al. 1985; Morales et al. 1993) and of Ca2+ channels from boar sperm plasma membranes (Cox and Peterson 1989).
Obtaining electrophysiological recordings directly in sperm to study their ion channels was exceedingly difficult for a long time (Darszon et al. 1999; Guerrero et al. 1987; Jimenez-Gonzalez et al. 2006; Kirichok and Lishko 2011; Ren and Xia 2010; Weyand et al. 1994). Achieving whole-cell patch-clamp recordings became more feasible when Kirichok et al. (2006) were able to seal the cytoplasmic droplet of mouse epididymal spermatozoa and then mature human spermatozoa (Kirichok and Lishko 2011). This novel strategy is allowing the characterization of sperm-specific channels such as CatSper (Kirichok et al. 2006) and SLO3 (Navarro et al. 2007; Santi et al. 2010; Schreiber et al. 1998; Zeng et al. 2011), and of sperm anion channels that are present in other cell types (Orta et al. 2012; Ferrera et al. 2010). Additionally, a voltage-sensitive H+ channel involved in the intracellular pH (pHi) regulation in human sperm and less importantly in mouse sperm (Kirichok and Lishko 2011), and ATP-gated channels of the purinergic family, P2X2, in mouse epididymal sperm have been recorded (Navarro et al. 2011). Ionic currents with properties consistent with TRPM8 channels were recorded in testicular sperm (Gibbs et al. 2011; Martinez-Lopez et al. 2011). Intracellular Ca2+ concentration [Ca2+] i imaging experiments at different temperatures and membrane potential (E m) measurements suggested TRPM8-like channels are present in more mature mouse and human sperm (De Blas et al. 2009; Martinez-Lopez et al. 2011).
Alternatively, whole-cell recordings have now been obtained directly patching the head of human spermatozoa using a modification of the perforated patch-clamp strategy . Employing this approach, Cl− currents displaying characteristics associated with Ca2+-dependent Cl− channels were documented in human spermatozoa (Orta et al. 2012).
Research in past years has established that in most cells Cl− is actively transported and not at electrochemical equilibrium. As a result, Cl− can participate in signaling and perform work, an important matter when considering the functional roles of anion channels and transporters, in addition to their more established participation in fluid secretion and volume regulation (Duran et al. 2012). In spermatozoa, as in other cells, Cl− is the main anion and is involved in volume regulation and osmotic stress protection (Cooper and Yeung 2007; Furst et al. 2002; Yeung et al. 2005). Capacitation, a maturational process and the AR, a unique exocytotic event essential for fertilization, both discussed next, are significantly affected in mouse and human spermatozoa when external Cl− concentrations are lowered (Chen et al. 2009; Figueiras-Fierro et al. 2013; Orta et al. 2012; Wertheimer et al. 2008; Yeung and Cooper 2008). Although these findings suggest that Cl− plays a relevant role in sperm physiology, not much is known about its transport across the membrane of this fundamental cell. This review summarizes information about Cl− channels and transporters for which evidence suggests their presence in sperm and their involvement in important sperm functions such as epididymal maturation, capacitation, motility, and the AR.
2 Maturation During Epididymal Transit
The epididymis is a specialized duct of the male reproductive system that fulfills four important functions for spermatozoa: transport, concentration, maturation, and storage (Turner 2008). The movement of sperm through the epididymis involves hydrostatic pressure and smooth muscle contractions. Depending on the species, sperm transit through the epididymis ranges from 2 to 13 days (Turner 2008). The epididymis is divided into three main sections: caput, corpus, and cauda, proximal to distal to the exit from the testis. Sperm from the caput section are immotile and lack important characteristics required for fertilization. In contrast, sperm obtained from the cauda have the highest fertilizing capacity. The osmolarity encountered by sperm during transit into the epididymis increases from 280 (in the rete testis fluid) to up to 400 mmol/kg (in the cauda epididymis fluid) (Yeung et al. 2006). On ejaculation into the female reproductive tract, spermatozoa experience hypo-osmotic stress, which is counterbalanced through the process known as regulatory volume decrease (RVD) involving influx and efflux of water and osmolytes (Yeung et al. 2006). RVD capability is acquired during epididymal transit , and sperm from the cauda exhibit the greatest RVD capacity. Results indicating the role of K+ channels during sperm RVD at the time of epididymal maturation suggest a parallel involvement of Cl− channels to compensate the positive charges and maintain electroneutrality (Cooper and Yeung 2007). The molecular identity of the Cl− channels involved in volume regulation is not established. Candidates such as ClC-2 (CLCN2) and ClC-3 (CLCN3) have been proposed to play a role in somatic cells (Furst et al. 2002; Nilius and Droogmans 2003); however, their function is still controversial (Sardini et al. 2003). Interestingly, CLCN3 was detected by Western blot and localized to the sperm tail by immunofluorescence (Yeung et al. 2005). Although the function of K+ and Cl− channels in RVD is still under study, the expression of such channels in sperm from several species suggests they may play an important role during epididymal maturation, a matter that awaits further research.
3 Motility
Sperm motility is activated when spermatozoa enter the female tract. Motility is one of the most important functions carried out by the sperm because it is essential to achieve fertilization. Indeed, several sperm motility defects can cause male sterility (e.g., sAC, PKA, sNHE, GAPDHs, CatSper, PMCA4, SLO3) (Esposito et al. 2004; Miki et al. 2004; Nolan et al. 2004; Okunade et al. 2004; Quill et al. 2001; Ren et al. 2001; Santi et al. 2010; Wang et al. 2007; Zeng et al. 2011). Sperm motility is driven by the flagellum, an appendage with an ultrastructure very similar to that of cilia. The axoneme is the principal structure that propels a flagellum (Lindemann and Goltz 1988); it is composed of a particular arrangement of microtubules, usually in the configuration of nine doublets surrounding a central pair. Movement results by repetitive cycles of flagellar bending, arising from microtubule sliding using the force generated by dynein ATPases whose activity is modulated by pH, ATP, ADP, Ca2+, and phosphorylation (Christen et al. 1983; Lindemann and Goltz 1988). Ion transport that supports and controls flagellar beating plays key roles in sperm motility regulation (Guerrero et al. 2011; Kaupp et al. 2008).
Upon ejaculation, sperm initiate motility with a relatively low-amplitude flagellar beat known as activated motility (Wennemuth et al. 2003). The stimulation of the sperm soluble adenylate cyclase by HCO3 − and the consequent cAMP/PKA stimulation is required for the activated motility (Carlson et al. 2007; Esposito et al. 2004; Hess et al. 2005; Nolan et al. 2004; Xie et al. 2006). After some time (varying according to species) in the female tract, spermatozoa become hyperactivated, displaying vigorous asymmetrical flagellar beating with large amplitude and high curvature. Hyperactivation helps sperm to detach from temporary binding sites along the female genital tract and to penetrate the extracellular matrix of cumulus cells and the zona pellucida (ZP) surrounding the oocyte (Suarez 2008). The mechanisms involved in hyperactivation are not well understood; however, it is known that a [Ca2+] i rise mediated by CatSper channels is required for hyperactivation. This Ca2+ channel, only present in the sperm flagella, is weakly voltage dependent and activated by an increase in pHi (Kirichok et al. 2006; Ren et al. 2001). CatSper null male mice are infertile mainly because of a failure to hyperactivate (Carlson et al. 2005; Carlson et al. 2003; Quill et al. 2001; Ren et al. 2001). It has been proposed that the hyperpolarization of the sperm plasma associated with capacitation increases the driving force for Ca2+, facilitating Ca2+ influx through CatSper channels during cytosolic alkalinization (Navarro et al. 2007).
4 Capacitation
A defined period of time in the female genital tract is necessary for mammalian spermatozoa to acquire their ability to fertilize (Austin 1952; Chang 1951). Altogether the set of changes required for this maturation process is called capacitation and involves the development of a distinctive sperm motility pattern known as hyperactivation, and the sperm capacity to undergo the AR, an exocytotic event that allows the sperm to fertilize the egg. During sperm capacitation PKA is activated (Harrison 2004), leading to tyrosine phosphorylation increases (Visconti et al. 1995a, b); pHi (Zeng et al. 1995) and [Ca2+] i elevate (Baldi et al. 1991; Breitbart 2003; DasGupta et al. 1993; Suarez et al. 1993; Xia and Ren 2009); plasma membrane composition and organization are modified (Cross 1998; Davis 1981; Gadella and Harrison 2000; Go and Wolf 1983; Travis and Kopf 2002; Visconti et al. 1999); and the cell E m is hyperpolarized in the mouse and other species (Arnoult et al. 1999; Demarco et al. 2003; Munoz-Garay et al. 2001; Zeng et al. 1995).
4.1 Membrane Potential Changes During Sperm Capacitation
It is yet not fully understood how and why E m is hyperpolarized in some mammalian sperm species. Indeed, hyperpolarization is important in mouse, bovine, and equine sperm capacitation (Arnoult et al. 1996; Arnoult et al. 1999; De La Vega-Beltran et al. 2012; Demarco et al. 2003; McPartlin et al. 2011; Munoz-Garay et al. 2001; Zeng et al. 1995), although it has not been demonstrated in human sperm. The sperm resting E m is relatively depolarized in most mammalian sperm (between −30 and −40 mV) (De La Vega-Beltran et al. 2012; Demarco et al. 2003; Espinosa and Darszon 1995; Hernandez-Gonzalez et al. 2006; McPartlin et al. 2011; Munoz-Garay et al. 2001; Santi et al. 2010; Zeng et al. 1995). Sperm populations are very heterogeneous and only a fraction of the cells capacitate in vitro (~30 %); therefore, the average E m values must be cautiously considered. Capacitated mouse spermatozoa display an average E m approximately −60 mV. Indeed, when Arnoult et al. (Arnoult et al. 1999) measured E m in individual spermatozoa using di-8-ANEPPS, a voltage-sensitive dye, they documented that capacitated sperm populations consisted of at least two groups: one hyperpolarized (~−80 mV), possibly representing capacitated sperm, and another of noncapacitated sperm with a resting E m approximately −43 mV. Various findings suggest that hyperpolarization is essential for sperm to acquire the ability to undergo a physiological AR. For instance, carrying out capacitation in the presence of high external KCl significantly reduces the ZP-induced mouse sperm AR (Arnoult et al. 1999; De La Vega-Beltran et al. 2012; Zeng et al. 1995). These observations lead to the proposal that an E m hyperpolarization is important for capacitation and thus is required for the AR.
Initially the role of the capacitation-associated hyperpolarization was thought to be needed to remove inactivation from T-type voltage-dependent Ca2+ channels (CaV3), which could then be activated by physiological agonists (e.g., ZP) to culminate with the induction of the AR (Arnoult et al. 1996; Arnoult et al. 1999; Santi et al. 1996; Zeng et al. 1995). Recent evidence suggests that hyperpolarization of the sperm plasma membrane is necessary and sufficient to prepare sperm for the AR (De la Vega et al. 2012). Even though the molecular entities and the mechanisms responsible for the hyperpolarization are not yet established, it could be the result of (1) an increase in K+ permeability caused by the activation of K+-selective channels, and (2) a reduction of Na+ permeability by decreasing the activity of Na+ channels. In this context, the regulation and activity of Cl− permeability through Cl− channels and transporters could also play a direct or indirect role in the regulation of the sperm plasma E m.
5 The Acrosome Reaction
The sperm head contains a large secretory vesicle located at its posterior end called the acrosome (Yanagimachi 1998). Spermatozoa of many species need to undergo the fusion of their single acrosome to the plasma membrane to be able to fertilize the female gamete. This process, called the AR, is now believed to occur in multiple steps. As multiple fusion points between the acrosomal and plasma membrane are involved in the AR, plasma membrane–outer acrosome hybrid vesicles are liberated, resulting in the release of the acrosomal content (Buffone et al. 2012). The fusion machinery involved in this reaction is regulated by Ca2+ and similar to that found in many neuroendocrinal secretory cells (Bello et al. 2012; Castillo Bennett et al. 2010). Notably, where and what triggers the physiologically relevant AR is being reevaluated (Inoue et al. 2011; Jin et al. 2011; Visconti and Florman 2010; Yanagimachi 1998).
In this context ZP and progesterone, as well as other AR inducers, require reexamination to establish their physiological relevance.
Various transduction pathways are required to converge for the ZP-induced AR to occur, and complex [Ca2+] i changes are involved (for review, see Mayorga et al. 2007). The physiologically relevant AR changes in [Ca2+] i include external and internal Ca2+ sources (Breitbart et al. 2010; Costello et al. 2009; Darszon et al. 2011; Florman et al. 2008). At the present time three different Ca2+ channels are thought to mediate the [Ca2+] i responses associated to the AR. They appear to be functionally linked in a manner that is not fully understood (Darszon et al. 2011; Florman et al. 2008; Publicover et al. 2007). Early on it was thought that voltage-dependent Ca2+ (CaV) channels were involved in the initial [Ca2+] i increase detected during the AR induced by ZP in mouse sperm, taking into account their pharmacology and that of this reaction (Darszon et al. 2011). CaV3.2 was the most likely CaV candidate to participate in the mouse AR (Arnoult et al. 1996; Escoffier et al. 2007; Lievano et al. 1996; Trevino et al. 2004). However, CaV3.1 and 3.2 knockout mice are fertile (Stamboulian et al. 2004), and CaV currents, although recorded in testicular sperm, were not detected in epididymal sperm (Martinez-Lopez et al. 2009; Ren and Xia 2010). These results raised doubts about the participation of CaV3 channels in the mouse sperm AR, although solid immunological data demonstrate their presence (Escoffier et al. 2007; Trevino et al. 2004).
A sustained [Ca2+] i increase lasting up to minutes is associated with the AR. Evidence indicates internal Ca2+ stores (i.e., the acrosome) participate releasing Ca2+ through the IP3 receptor, the second type of Ca2+ channel involved in the AR, which is activated as a consequence of IP3 production during the AR (Darszon et al. 2011; Florman et al. 2008; Mayorga et al. 2007; Publicover et al. 2007). Plasma membrane Ca2+ channels (SOCS) activate as Ca2+ store emptying occurs, causing the sustained [Ca2+] i increase. Several components such as STIM, ORAI, and TRPCs may constitute SOCS (Moreno and Vaca 2011). STIM and ORAI have been suggested to be present in human and mouse sperm and contribute to the sustained [Ca2+] i elevation leading to the AR (Costello et al. 2009; Darszon et al. 2012).
As discussed, Ca2+ transport plays a fundamental role in the AR. Much less is known about how Cl− movements influence this event. Interestingly, niflumic acid (NFA), best known as a Ca2+-activated Cl− channel inhibitor, was reported a long time ago to block the first Cl− single-channel activity recorded in mammalian sperm as well as the AR induced by solubilized ZP, progesterone, and GABA in mouse sperm (Espinosa et al. 1998). NFA also partially inhibited a Ca2+-induced hyperpolarization partially driven by Cl− in mouse spermatozoa (Espinosa et al. 1998). Anion channel blockers such as NFA, for example, DIDS, also inhibit the mouse and human sperm AR, as well as Cl− channels detected in these cells (Espinosa and Darszon 1995; Espinosa et al. 1998; Figueiras-Fierro et al. 2013; Orta et al. 2012).
Considering the reevaluation of the site(s) and mechanisms that trigger the AR requires also a reexamination of the evidence involving neurotransmitter receptors in this process. These matters are discussed in the section on the GABAA and glycine receptors, which are particularly relevant to this review as they mediate Cl− fluxes.
6 Cl− Channels and Transporters Linked to Sperm Physiology
In a recent paper our group reported that when sperm are incubated in Cl−-free media, most of the capacitation-associated processes are impaired (Hernandez-Gonzalez et al. 2007; Wertheimer et al. 2008). In this condition, increase in tyrosine phosphorylation and hyperpolarization of the sperm E m are not observed. Consistently, sperm did not hyperactivate (discussed below), were unable to undergo the AR, and failed to fertilize in an in vitro assay. Although in the absence of Cl− cAMP agonists rescued phosphorylation events, this condition was not sufficient to allow the sperm to fertilize in vitro. These findings highlight the importance of Cl− homeostasis in sperm during capacitation, and suggest that one or more Cl− transport systems are present in sperm. The identity of the specific sperm Cl− transporters involved in capacitation is still unclear.
The activity of all Cl− transporters present in a particular cell type defines its [Cl−] i levels. These transporters can be divided in two categories: Cl− channels and specialized Cl− carriers (Jentsch et al. 2005; Nilius and Droogmans 2003). Cl− channels are distributed in four groups: (1) CFTR channels; (2) the γ-aminobutyric (GABA)-gated and related glycine-gated neurotransmitter receptors; (3) Ca2+-activated Cl− channels; and (4) CLC channels. Cl− carriers couple the transport of Cl− to the movement of another ion in either opposite direction (antiporter) or in the same direction (cotransporter or symporter). Cl− carriers are classified in two main families: (1) the electro-neutral cation Cl− cotransporter family and (2) the electro-neutral Cl−/HCO3 − exchanger family.
6.1 CFTR Channels
The ABC transporter family has a unique member, the cystic fibrosis transmembrane conductance regulator (CFTR), an anion channel modulated by cAMP/PKA and ATP. The CFTR consists of two membrane-spanning domains (MSDs), two nucleotide-binding domains (NBDs), and a regulatory (R) domain. The MSDs have six transmembrane helices each, which are linked by the regulatory domain. In cells possessing either endogenous or recombinant CFTR, the channel displays the following anionic selectivity sequence: Br− > Cl− > I− > F− (Anderson et al. 1991). The channel pore is formed by the MSDs, whereas the gating activity is related to ATP hydrolysis by the NBDs and phosphorylation of the R domain (Sheppard and Welsh 1999). Mutations in CFTR cause cystic fibrosis (CF), an autosomic recessive genetic disease characterized by abnormal transport of Cl− and HCO3 − leading to viscous secretions in many epithelial cells, but especially the lungs, pancreas, liver, and intestine.
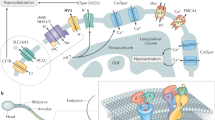
CFTR mutations affect male fertility; as on average 95 % of male CF patients have congenital bilateral absence of the vas deferens, making them infertile. In addition, other mechanisms related to sperm physiology may be affected by CFTR mutations, leading to infertility in CF (Popli and Stewart 2007). Supporting this notion, it has been found that fertility in uremic patients may be reduced through alterations in CFTR expression (Xu et al. 2012). CFTR has been shown to be present in both human and mouse sperm by our group and others, using specific antibodies (Chan et al. 2006; Hernandez-Gonzalez et al. 2007; Li et al. 2010; Xu et al. 2007). Xu et al. (2007) showed, using CFTR inhibitors and specific antibodies, as well as heterozygous CFTR mutants, that sperm capacitation and the associated HCO3 − transport are significantly reduced when compared to mice in normal fertilizing conditions. CFTR function has also been identified electrophysiologically as ATP-dependent Cl− currents that are stimulated by cAMP, cGMP, and genistein, and inhibited by DPC and CFTRinh-172 in whole-cell clamp recordings from testicular and epididymal mouse sperm (Figueiras-Fierro et al. 2013). The biophysical and pharmacological properties of CFTR recorded from epididymal mice spermatozoa, as well as its role in the AR, are shown in Fig. 6.1. Moreover, this particular Cl− current is absent in testicular sperm from mice displaying the most common variant of the CF mutation, the loss-of-function mutation of the CFTR gene known as ∆F508. All these findings support the idea that CFTR is present in mature spermatozoa and that it is involved in the capacitation events.
Cystic fibrosis transmembrane conductance regulator (CFTR) channels are functional in epididymal mouse spermatozoa. (a) Representative whole-cell patch-clamp currents recorded by applying voltage pulses from a holding potential of −40 mV to test potentials ranging from 100 to −80 mV in 20-mV steps. The protocol used for eliciting CFTR currents is shown between a and c traces. Sperm were exposed to recording solutions where the primary ion is Cl−. Control Cl− currents (filled squares) were significantly stimulated by extracellular db-cAMP (100 μM; filled circles) and inhibited following the addition to the external media of the specific inhibitor, CFTRinh-172 (5 μM; filled triangles db-cAMP + CFTRinh-172). CFTRinh-172 inhibition was partially reversible (hollow squares). The effects of agonist and CFTRinh-172 were recorded in the presence of niflumic acid (NFA) (50 μM), to eliminate the contribution of other Cl− channels. (b) I–V relationship of the currents in a. CFTR currents similar to those detected in epididymal sperm were also present in testicular sperm. (c) CFTR inhibition during capacitation significantly inhibited acrosome reaction (AR). Mouse sperm were capacitated in medium supplemented with bovine serum albumin and NaHCO3, and AR was induced with ZP or A23187 (Ca2+ ionophore). The presence of DPC, a classic CFTR inhibitor, during sperm capacitation decreased the ZP-induced AR if added during capacitation but not if added during AR induction. For a and b, symbols represent the mean ± SEM of four experiments; some SE bars were smaller than the symbols. The currents were normalized with respect to the stimulated Cl− current at 100 mV. For c, data are normalized with respect to spontaneous AR and presented as the mean ± SEM with n ≥ 3
How E m is regulated by Cl− and other anions is still poorly understood. As CFTR is mainly a Cl− channel, it possibly participates in regulation of the resting E m; however, Cl− substitution by nonpermeable anions (e.g., gluconate or methanesulfonate) does not modify it. On the other hand, this procedure inhibits hyperpolarization development during capacitation (Hernandez-Gonzalez et al. 2007). Findings supporting this idea in mouse sperm are (1) adding 250 μM of the CFTR antagonist DPC (diphenylamine-2-carboxylic acid) inhibits hyperpolarization associated with capacitation and also the AR induced by sZP, without modifying tyrosine phosphorylation levels; (2) the CFTR agonist genistein (5–10 μM) hyperpolarizes noncapacitated spermatozoa; and (3) addition of permeable analogues of cAMP to noncapacitated sperm elevates [Cl−] i (Hernandez-Gonzalez et al. 2007). CFTR channels are also known to interact with and regulate other ion channels (i.e., epithelial Na+ channels, ENaCs ) and transporters (i.e., the Cl−/HCO3 − exchanger, SCL family) (Berdiev et al. 2009; Konig et al. 2001; Kunzelmann and Schreiber 1999; Perez-Cornejo and Arreola 2004). The interaction of CFTR with the Cl−/HCO3− exchangers is important to explain its role in pHi regulation (discussed in Sect. 6.6.6). Of particular relevance to understand how CFTR influences the resting E m is its interaction with ENaC. As the sperm resting E m is mildly depolarized (~ −35 mV) and thus shifted from the K+ equilibrium potential, it cannot be explained mainly by a high K+ permeability through K+ channels, as it usually happens in many cells. Just a 10 % contribution of Na+ permeability would explain the observed resting E m in noncapacitated sperm, and their inhibition during capacitation would contribute to the observed hyperpolarization (see Hernandez-Gonzalez et al. 2006; Hernandez-Gonzalez et al. 2007). CFTR can downregulate ENaCs through mechanisms still not fully understood (Konig et al. 2001), and the CFTR agonist genistein hyperpolarizes sperm E m (Hernandez-Gonzalez et al. 2007) and diminishes [Na+] i (Escoffier et al. 2012). In summary, CFTR is important in sperm physiology regulating pHi and E m in the resting and capacitating conditions, directly through its Cl− and anion permeability or indirectly through its interaction with ENaC and Cl−/HCO3 − exchangers.
6.2 GABA and Glycine Receptors
GABAA receptors are ligand-gated anion channels selective for Cl− ions, which mediate inhibitory neurotransmission in the central nervous system (CNS). These receptors are pentameric and composed of different subunits. They were first identified pharmacologically as being activated by GABA and the selective agonist muscimol, blocked by bicuculline and picrotoxin, and modulated by benzodiazepines, barbiturates, and certain other central nervous system (CNS) depressants (Macdonald and Olsen 1994; Sieghart 1995). GABAA receptors are also found outside the CNS, in tissues such as liver, lung, and immune cells (Sigel and Steinmann 2012). Polymerase chain reaction (PCR) and immunocytochemistry studies revealed the presence of GABAA and GABAB receptors in spermatogenic cells, and patch-clamp studies showed that GABA application to round spermatogenic cells induced an inward Cl− current (Kanbara et al. 2011). The presence and function of GABAA receptors in sperm from different species have been explored by several laboratories (Jin et al. 2009; Meizel 1997; Puente et al. 2011; Wistrom and Meizel 1993). For example, Ritta et al. established that this neurotransmitter plays a role in the regulation of motility in bovine and human sperm (Ritta et al. 2004; Ritta et al. 1998). In rat sperm, GABA and progesterone accelerated the process of capacitation and hyperactivated motility, effects that were inhibited by bicuculline and picrotoxin (Jin et al. 2009). Additionally, it has been shown that GABA can induce the AR (Puente et al. 2011; Shi et al. 1997) and also that GABAA receptors can modulate the response to progesterone in these cells (Hu et al. 2002; Ritta et al. 1998; Shi and Roldan 1995; Turner et al. 1994).
Glycine receptors are also heteropentameric Cl− channels that mediate inhibitory transmission in the CNS, although they are also found in retina and macrophages (Webb and Lynch 2007). The alkaloid strychnine has been used as a very specific antagonist for these receptors. The presence and functionality of glycine receptors in sperm have been studied especially in Meizel’s laboratory. They reported the expression of different glycine receptor isoforms in sperm from human, porcine, mouse, and hamster using both immunocytochemistry and Western blot analysis (Bray et al. 2002; Kumar and Meizel 2008; Llanos et al. 2001; Meizel and Son 2005; Melendrez and Meizel 1995; Sato et al. 2000). These receptors were detected in the head and flagellum, suggesting distinct roles at different cell locations. For example, antibodies against glycine receptors A1 and A2 inhibited the ZP3-induced AR in human sperm (Bray et al. 2002).
Compounds such as glycine, GABA, and acetylcholine were reported many years ago to induce the AR; however, the physiological significance of these observations was unclear, considering that the accepted physiological inductor for this reaction was ZP3. Recent findings have challenged this paradigm, and where, when, and what induces the AR are open questions again (Inoue et al. 2011; Jin et al. 2011; Kunzelmann et al. 2011).
6.3 Ca2+-Activated Cl− Channels (CaCCs)
In many cell types, volume control and secretion are critical (i.e., reproductive tract smooth muscle cells, oviduct and ductus epididymis cells, spermatids, epithelial cells in exocrine glands and trachea, airway, and vascular smooth muscle cells), and spermatozoa are not an exception. Usually these cells possess Ca2+-activated Cl− channels (CaCCs) that display similar biophysical and molecular features (Hartzell et al. 2005; Huang et al. 2009). Such currents were initially documented in Xenopus oocytes (Miledi 1982) but have now been recorded in many cell types.
An elevation of [Ca2+] i resulting from release from intracellular stores or influx through plasma membrane channels activates CaCCs. In spite of the frequent presence of CaCCs in cells, their molecular identity is not fully known. Candidates considered for CaCCs are bestrophins, tweety, and CLCA. However, all of them failed to reproduce the native behavior of CaCCs when expressed in heterologous expression systems (Hartzell et al. 2009). Recently, using bioinformatics approaches, different research teams succeeded isolating and expressing genes in heterologous expression systems that elicit almost identical CaCCs currents to those reported in native cells. The identity of these molecules that seem to be the molecular basis of CaCCs are anoctamins (TMEM16) (Caputo et al. 2008; Ferrera et al. 2010; Schroeder et al. 2008; Yang et al. 2008). The term anoctamin refers to the property of being anion selective and having subunits with a putative topology consisting of eight transmembrane segments and cytosolic N- and C-termini (Galietta 2009). One of the main TMEM16 proteins whose activity strongly resembles that of CaCCs is TMEM16A, corresponding to anoctamin-1. The TMEM16/anoctamin family has nine more members named TMEM16B to -K, or anoctamin 2–10 (Galindo and Vacquier 2005). The biophysical characteristics among the members of this family of ion channels such as voltage dependence, selectivity, and conductance differ (Pifferi et al. 2009; Scudieri et al. 2012). Almost all show enzymatic properties in addition to the transport function, which has led to the speculation that they are multifunctional proteins, one of them being permeable to Cl− (Tian et al. 2012).
As mentioned, earlier electrophysiological evidence for the presence of Cl− channels in sperm had been gathered. The initial patch-clamp recordings directly on epididymal mouse sperm revealed an anion channel displaying biophysical properties and sensitivity to NFA, resembling to the Ca2+-dependent Cl− channels (Espinosa et al. 1998; Hogg et al. 1994). Thereafter, a Cl−-permeable channel showing long stable openings was documented in patch-clamp studies in the cell-attached mode in the human sperm. Distinct channel clustering and activity was detected in different sperm head regions whose functional significance awaits determination (Jimenez-Gonzalez et al. 2007).
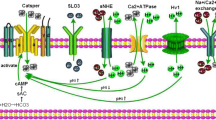
More recently, using a modified perforated patch-clamp technique, whole-cell recordings of the human mature spermatozoa head revealed that an important component of their Cl− currents is in fact caused by CaCCs, possibly of the TMEM16A type (Orta et al. 2012). Supporting evidence was based on the biophysical properties and in the pharmacology profile of the recorded currents. The typical results of such experiments characterizing biophysically and pharmacologically CaCCs in epididymal human spermatozoa are shown in Fig. 6.2. One of the most specific antagonists of TMEM16A channels, the drug TMEM16Ainh (20 μM), inhibited these currents and reduced ~80 % of the AR. These results suggest the critical participation of these channels during the AR. Results supporting this hypothesis are shown in Fig. 6.3. It is known that during the AR there are large [Ca2+] i changes leading to profound modifications in the sperm head morphology involving acrosome swelling and a decrease in regulatory volume; therefore, CaCCs channels may participate in this event (Zanetti and Mayorga 2009). Blockage of CaCCs by NFA, DIDS, and TMEM16Ainh may affect the reduction in regulatory volume, which in turn seems to be important to regulate acrosomal–plasma membrane distance, essential for acrosomal exocytosis (Zanetti and Mayorga 2009).
Ca2+-activated Cl− channel (CaCC) currents in epididymal human spermatozoa. (a) Whole-cell CaCC currents recorded from a human spermatozoa. Currents were obtained at a holding potential of 0 mV with the indicated voltage step protocol (top panel). The control currents (Control) were recorded before and after exposure to 10 μM TMEM16Ainh (bottom panel). (b) I–V plot of the blockade of the CaCC currents ([Ca2+] i = 250 nM) by 10 μM NFA and 10 μM TMEM16Ainh. Currents were normalized with respect to maximal current in control measurements at the end of each voltage pulse. (c) Dose-dependent blockade of CaCC currents by TMEM16Ainh. Current amplitudes were measured at +100 mV by averaging seven to nine original current traces and normalized with respect to the maximal blocked fraction. (d) Concentration–response curve at different [Ca2+] i on the macroscopic inward and outward Cl− currents obtained from seals on the cytoplasmic droplet against the current obtained at 1,000 nM [Ca2+] i recorded at +100 mV. The continuous lines represent the data fitted to the Hill equation with the following parameters: K d = 163 nM and n H = 1.9 at +100 mV. For b and c, data represent the mean ± SEM with n = 6; for d, data represent mean ± SEM with n = 7
The rhZP3-induced AR in human spermatozoa is inhibited by CaCC/TMEM16Ainh. Motile human spermatozoa were obtained by the swim-up technique and capacitated during 5 h. Sperm populations were preincubated for 15 min with different NFA, DIDS, and TMEM16Ainh concentrations. The AR was induced with rhZP3 (10 ng μl−1). Cells were fixed with cold methanol and the acrosomal status evaluated after staining sperm with FITC-PSA. Acrosomal reaction was expressed as an index (ARI = percentage of AR normalized against the maximum AR with ionomycin) and was used to estimate the percentage of AR inhibition. NFA (a) and DIDS (b), two CaCCs blockers, inhibited 90 % of the AR. TMEM16Ainh (c) blocked approximately 80 %, indicating that TMEM16A channels may have an essential contribution in the AR. For all data, error bars represent mean ± SEM with n = 4–6. Statistical comparisons according to Student’s unpaired t test indicated *P < 0.05; **P < 0.01; ***P < 0.001 versus spermatozoa incubated with 0.1 % DMSO + 10 ng μl−1 of rhZP3
Evidence gathered recently has shown that CaCCS and voltage and Ca2+-gated K+ channels (BKCa, Slo1, or KCa1.1) have some unexpected similarities regarding their pharmacological properties (Greenwood and Leblanc 2007; Sones et al. 2009). Classical Cl− channel inhibitors with different structures such as anthracene-9-carboxylate, NFA, and ethacrynic acid also behave as BKCa agonists (Greenwood and Large 1995; Ottolia and Toro 1994; Toma et al. 1996). The potent ability to inhibit the AR by classical anion channel blockers such as NFA may be explained by a combination of the effects of this drug on BKCa and CaCCs, as BKCa channels are also present in mammalian sperm (Rossato et al. 2001; Wu et al. 1998).
Notably, it has also been shown that TMEM16A channels may increase their permeability to HCO3 − on raising [Ca2+] i through Ca2+/calmodulin modulation (Jung et al. 2013). This finding is relevant to sperm physiology because HCO3 − plays a key role in cAMP production and pHi regulation (Visconti et al. 2011). It has also been shown that CaCCa can be modulated through the activity of other ion channels also present in spermatozoa (i.e., purinergic receptors) (Wang et al. 2013). Taking these facts into account, it is likely that new evidence of their critical relevance will be gathered in the future.
In summary, CaCCs significantly influence sperm physiology and are likely players in the solubilized ZP-induced AR. It will be interesting to further investigate their role in sperm motility, as inklings of their involvement in sea urchin sperm chemotaxis have been reported (Alvarez et al. 2012; Guerrero et al. 2013; Ingermann et al. 2008; Wood et al. 2003; Wood et al. 2007).
6.4 Voltage-Dependent Anion Channels (VDACs)
VDACs are porins, usually located on the outer mitochondrial membrane (Liu et al. 2010). They seem to be critical for mitochondria metabolism and regulation of apoptosis as they are permeant to small hydrophilic molecules. In this sense, they have been described as molecules able to “regulate cell life and death” (Shoshan-Barmatz et al. 2010). They seem to be involved in the pathogenesis of a variety of processes such as cancer, neurodegenerative diseases, and ischemic-reperfusion injuries in the heart (Peixoto et al. 2012). VDAC2 is the main variant present in the male germline, although VDAC3 has also been found (Liu et al. 2010). VDACs have been localized not only in the mitochondrial sheath but also in the plasma membrane and outer dense fiber (Hinsch et al. 2004; Liu et al. 2009; Triphan et al. 2008). It has been reported recently that they might play a role in human fertility (Kwon et al. 2013). Consistent with this proposal, VDAC2 was found to be one of the putative sperm head membrane proteins that bind ZP (Petit et al. 2013).
6.5 Secondary Active Cl− Transporters
A [Cl−] i increase has been reported to occur during capacitation (Hernandez-Gonzalez et al. 2007; Meizel and Turner 1996), and it is possible that several Cl− transport systems participate in this process. Under physiological conditions electro-neutral carriers such as NCC, NKCC support transport of Cl− into the cell and KCC outside the cell (Russell 2000). Pharmacological approaches have been used to test the participation of these carriers during sperm capacitation. Stilbenes such as DIDS and SITS, which are general Cl− transport blockers, reduced sperm capacitation parameters to similar levels as those observed in the absence of Cl−. Two specific inhibitors (bumetanide and furosemide) for NKCC blocked the increase in tyrosine phosphorylation, hyperactivation, and the ability of the sperm to fertilize in vitro, but the concentration required to observe these effects was higher than that specifically reported to inhibit NKCC (Garg et al. 2007; Russell 2000). NKCC function requires the presence of Na+, K+, and Cl−, but tyrosine phosphorylation is affected in the absence of Na+ and Cl− but not K+, suggesting that bumetanide and furosemide may be acting through a target other than NKCC. It is worth noting that the ZP-induced AR depended on the presence of the three ions and was inhibited at much lower concentrations of bumetanide, suggesting that NKCC might have a role in the preparation of the sperm for the physiologically induced AR. NKCC1 transcripts were detected in spermatids, and male mouse null mutants of this protein have defects in spermatogenesis and are infertile (Pace et al. 2000). Thiazide, a specific NCC inhibitor, did not interfere with capacitation-associated processes.
6.6 Cl−/HCO3 − Exchangers
Cl−/HCO3 − transporters are proteins that exchange Cl− for HCO3 − in either direction. HCO3 − regulation is particularly important in spermatozoa because it activates cAMP synthesis by the atypical soluble adenylyl cyclase present in these cells (Hess et al. 2005; Okamura et al. 1985). The specific carriers responsible for HCO3 − transport have not yet been fully defined. Experiments from our group suggest that Na+/HCO3 − cotransporters allow HCO3 − influx into mouse sperm (Demarco et al. 2003). However, other HCO3 − transport systems can also play a role in the control of HCO3 − levels in sperm. In particular, Cl−/HCO3 − exchangers have been proposed to be involved in HCO3 − homeostasis. These exchangers also influence pHi, cell volume, and E m through their contribution to determine the Cl− gradient. Two superfamilies group the Cl−/HCO3 − exchangers, SLC4 and SLC26; they have different anion selectivity and unique tissue distribution. The SLC4 superfamily is composed of 3 genes (AE1, AE2, and AE3), each of them represented by more than one alternative spliced sequence. The SLC26 gene superfamily consists of 11 genes but only SLC26A3, SLC26A4, and SLC26A6 have Cl−/HCO3 − exchange activity.
The AE2 gene is the only member from the SLC4 superfamily reported in spermatogenic cells. This gene has five splice isoforms (AE2a, AE2b1, AE2b2, AE2c1, AE2c2), and mice lacking their expression die before weaning of severely retarded development (Gawenis et al. 2004). Mice expressing only the AE2c isoform survive but they are infertile and exhibit testicular dysplasia, consistent with the observation that AE2 is highly expressed in the testis (Medina et al. 2003).
Recent Western blot and immunofluorescence results from several laboratories, including ours, indicate the presence of SLC26A3 and SLC26A6 in the sperm midpiece (Chan et al. 2009; Chavez et al. 2011; Chen et al. 2009). Chavez et al. (2011), also provided evidence that these transporters co-precipitate with CFTR and that tenidap, a specific SLC26A3 inhibitor, blocked the capacitation-associated hyperpolarization and the ZP-induced AR. However, tenidap did not affect the activation of a cAMP pathway or the increase in tyrosine phosphorylation, suggesting that these transporters are not directly involved in the regulation of the soluble adenylate cyclase.
7 Final Remarks
Work reported in recent years corroborates the significant repercussions of ion channels in sperm maturation, capacitation, and the AR. However, our knowledge regarding the molecular mechanisms regulating these processes is still limited. The study of ion transport in spermatozoa, specially the use of electrophysiological techniques, has been a difficult enterprise because of their small size and the stiffness of their membrane. Fortunately, new patch-clamp strategies have improved our ability to study sperm ion channels. Although the inability of these cells to perform transcription and translation significantly complicates knocking down or expressing exogenous proteins, advances in the production of genetically modified mice have enhanced the identification of key proteins, processes, and mechanisms essential to sperm function. The increasing sensitivity and speed of single-cell ion-imaging strategies is helping to unravel sperm signaling networks and how ion channels participate in them. We hope the readers have become aware that the study of sperm anion transport requires our full attention, as it is deeply implicated in the physiology of this important cell. Enhancing our understanding of sperm ion transport will impact our capacity to preserve animal species and improve our control of fertility.
References
Alvarez L, Dai L, Friedrich BM, Kashikar ND, Gregor I, Pascal R, Kaupp UB (2012) The rate of change in Ca(2+) concentration controls sperm chemotaxis. J Cell Biol 196:653–663
Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ (1991) Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253:202–205
Arnoult C, Cardullo RA, Lemos JR, Florman HM (1996) Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci USA 93:13004–13009
Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, Florman HM (1999) Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc Natl Acad Sci USA 96:6757–6762
Austin CR (1952) The capacitation of the mammalian sperm. Nature (Lond) 170:326
Baker MA (2011) The omics revolution and our understanding of sperm cell biology. Asian J Androl 13:6–10
Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G (1991) Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl 12:323–330
Bello OD, Zanetti MN, Mayorga LS, Michaut MA (2012) RIM, Munc13, and Rab3A interplay in acrosomal exocytosis. Exp Cell Res 318:478–488
Berdiev BK, Qadri YJ, Benos DJ (2009) Assessment of the CFTR and ENaC association. Mol Biosyst 5:123–127
Bray C, Son JH, Kumar P, Harris JD, Meizel S (2002) A role for the human sperm glycine receptor/Cl(−) channel in the acrosome reaction initiated by recombinant ZP3. Biol Reprod 66:91–97
Breitbart H (2003) Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol (Noisy-le-grand) 49:321–327
Breitbart H, Rotman T, Rubinstein S, Etkovitz N (2010) Role and regulation of PI3K in sperm capacitation and the acrosome reaction. Mol Cell Endocrinol 314:234–238
Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL (2012) Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev 79:4–18
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322:590–594
Carlson AE, Hille B, Babcock DF (2007) External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol 312:183–192
Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, Babcock DF (2005) Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol Chem 280:32238–32244
Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF (2003) CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci USA 100:14864–14868
Castillo Bennett J, Roggero CM, Mancifesta FE, Mayorga LS (2010) Calcineurin-mediated dephosphorylation of synaptotagmin VI is necessary for acrosomal exocytosis. J Biol Chem 285:26269–26278
Chan HC, Ruan YC, He Q, Chen MH, Chen H, Xu WM, Chen WY, Xie C, Zhang XH, Zhou Z (2009) The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. J Physiol 587:2187–2195
Chan HC, Shi QX, Zhou CX, Wang XF, Xu WM, Chen WY, Chen AJ, Ni Y, Yuan YY (2006) Critical role of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Mol Cell Endocrinol 250:106–113
Chang MC (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature (Lond) 168:697–698
Chavez JC, Hernandez-Gonzalez EO, Wertheimer E, Visconti PE, Darszon A, Trevino CL (2011) Participation of the Cl−/HCO3 − exchangers SLC26A3 and SLC26A6, the Cl− channel CFTR and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol Reprod 86:1–14
Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, Tsang LL, Chung YW, Hoglund P, Chan HC, Shi QX (2009) Cl− is required for HCO3 − entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3 − exchanger (SLC26A3) and CFTR. Biol Reprod 80:115–123
Christen R, Schackmann RW, Shapiro BM (1983) Metabolism of sea urchin sperm. Interrelationships between intracellular pH, ATPase activity, and mitochondrial respiration. J Biol Chem 258:5392–5399
Cooper TG, Yeung CH (2007) Involvement of potassium and chloride channels and other transporters in volume regulation by spermatozoa. Curr Pharm Des 13:3222–3230
Costello S, Michelangeli F, Nash K, Lefievre L, Morris J, Machado-Oliveira G, Barratt C, Kirkman-Brown J, Publicover S (2009) Ca2+-stores in sperm: their identities and functions. Reproduction 138:425–437
Cox T, Peterson RN (1989) Identification of calcium-conducting channels in isolated boar sperm plasma membranes. Biochem Biophys Res Commun 161:162–168
Cross NL (1998) Role of cholesterol in sperm capacitation. Biol Reprod 59:7–11
Dacheux JL, Belleannee C, Guyonnet B, Labas V, Teixeira-Gomes AP, Ecroyd H, Druart X, Gatti JL, Dacheux F (2012) The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst Biol Reprod Med 58:197–210
Darszon A, Labarca P, Nishigaki T, Espinosa F (1999) Ion channels in sperm physiology. Physiol Rev 79:481–510
Darszon A, Nishigaki T, Beltran C, Trevino CL (2011) Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev 91:1305–1355
Darszon A, Sanchez-Cardenas C, Orta G, Sanchez-Tusie AA, Beltran C, Lopez-Gonzalez I, Granados-Gonzalez G, Trevino CL (2012) Are TRP channels involved in sperm development and function? Cell Tissue Res 349(3):749–764
DasGupta S, Mills CL, Fraser LR (1993) Ca(2+)-related changes in the capacitation state of human spermatozoa assessed by a chlortetracycline fluorescence assay. J Reprod Fertil 99:135–143
Davis BK (1981) Timing of fertilization in mammals: sperm cholesterol/phospholipid ratio as a determinant of the capacitation interval. Proc Natl Acad Sci USA 78:7560–7564
De Blas GA, Darszon A, Ocampo AY, Serrano CJ, Castellano LE, Hernandez-Gonzalez EO, Chirinos M, Larrea F, Beltran C, Trevino CL (2009) TRPM8, a versatile channel in human sperm. PLoS One 4:e6095
De La Vega-Beltran JL, Sanchez-Cardenas C, Krapf D, Hernandez-Gonzalez EO, Wertheimer E, Trevino CL, Visconti PE, Darszon A (2012) Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J Biol Chem 287:44384–44393
Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE (2003) Involvement of a Na+/HCO−3 cotransporter in mouse sperm capacitation. J Biol Chem 278:7001–7009
Duran C, Qu Z, Osunkoya AO, Cui Y, Hartzell HC (2012) ANOs 3-7 in the anoctamin/Tmem16 Cl− channel family are intracellular proteins. Am J Physiol Cell Physiol 302:C482–C493
Escoffier J, Boisseau S, Serres C, Chen CC, Kim D, Stamboulian S, Shin HS, Campbell KP, De Waard M, Arnoult C (2007) Expression, localization and functions in acrosome reaction and sperm motility of Ca(V)3.1 and Ca(V)3.2 channels in sperm cells: an evaluation from Ca(V)3.1 and Ca(V)3.2 deficient mice. J Cell Physiol 212:753–763
Escoffier J, Krapf D, Navarrete F, Darszon A, Visconti PE (2012) Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J Cell Sci 125:473–485
Espinosa F, Darszon A (1995) Mouse sperm membrane potential: changes induced by Ca2+. FEBS Lett 372:119–125
Espinosa F, de la Vega-Beltran JL, Lopez-Gonzalez I, Delgado R, Labarca P, Darszon A (1998) Mouse sperm patch-clamp recordings reveal single Cl− channels sensitive to niflumic acid, a blocker of the sperm acrosome reaction. FEBS Lett 426:47–51
Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA (2004) Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA 101:2993–2998
Ferrera L, Caputo A, Galietta LJ (2010) TMEM16A protein: a new identity for Ca(2+)-dependent Cl(-) channels. Physiology (Bethesda) 25:357–363
Figueiras-Fierro D, Acevedo JJ, Martinez-Lopez P, Escoffier J, Sepulveda FV, Balderas E, Orta G, Visconti PE, Darszon A (2013) Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm. J Cell Physiol 228:590–601
Florman HM, Jungnickel MK, Sutton KA (2008) Regulating the acrosome reaction. Int J Dev Biol 52:503–510
Furst J, Gschwentner M, Ritter M, Botta G, Jakab M, Mayer M, Garavaglia L, Bazzini C, Rodighiero S, Meyer G, Eichmuller S, Woll E, Paulmichl M (2002) Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch 444:1–25
Gadella BM, Harrison RA (2000) The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development (Camb) 127:2407–2420
Galietta LJ (2009) The TMEM16 protein family: a new class of chloride channels? Biophys J 97:3047–3053
Galindo BE, Vacquier VD (2005) Phylogeny of the TMEM16 protein family: some members are overexpressed in cancer. Int J Mol Med 16:919–924
Garg P, Martin CF, Elms SC, Gordon FJ, Wall SM, Garland CJ, Sutliff RL, O’Neill WC (2007) Effect of the Na-K-2Cl cotransporter NKCC1 on systemic blood pressure and smooth muscle tone. Am J Physiol Heart Circ Physiol 292:H2100–H2105
Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE (2004) Mice with a targeted disruption of the AE2 Cl−/HCO3 − exchanger are achlorhydric. J Biol Chem 279:30531–30539
Gibbs GM, Orta G, Reddy T, Koppers AJ, Martinez-Lopez P, Luis de la Vega-Beltran J, Lo JC, Veldhuis N, Jamsai D, McIntyre P, Darszon A, O’Bryan MK (2011) Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc Natl Acad Sci USA 108:7034–7039
Go KJ, Wolf DP (1983) The role of sterols in sperm capacitation. Adv Lipid Res 20:317–330
Greenwood IA, Large WA (1995) Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol 116:2939–2948
Greenwood IA, Leblanc N (2007) Overlapping pharmacology of Ca2+-activated Cl− and K+ channels. Trends Pharmacol Sci 28:1–5
Guerrero A, Carneiro J, Pimentel A, Wood CD, Corkidi G, Darszon A (2011) Strategies for locating the female gamete: the importance of measuring sperm trajectories in three spatial dimensions. Mol Hum Reprod 17:511–523
Guerrero A, Espinal J, Wood CD, Rendon JM, Carneiro J, Martinez-Mekler G, Darszon A (2013) Niflumic acid disrupts marine spermatozoan chemotaxis without impairing the spatiotemporal detection of chemoattractant gradients. J Cell Sci 126:1477–1487
Guerrero A, Sanchez JA, Darszon A (1987) Single-channel activity in sea urchin sperm revealed by the patch-clamp technique. FEBS Lett 220:295–298
Harrison RA (2004) Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol Reprod Dev 67:337–352
Hartzell C, Putzier I, Arreola J (2005) Calcium-activated chloride channels. Annu Rev Physiol 67:719–758
Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z (2009) Anoctamin/TMEM16 family members are Ca2+-activated Cl- channels. J Physiol 587(Pt 10):2127–2139
Hernandez-Gonzalez EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, Lopez-Gonzalez I, Demarco I, Wertheimer E, Darszon A, Visconti PE (2006) Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem 281:5623–5633
Hernandez-Gonzalez EO, Trevino CL, Castellano LE, de la Vega-Beltran JL, Ocampo AY, Wertheimer E, Visconti PE, Darszon A (2007) Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem 282:24397–24406
Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB (2005) The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9:249–259
Hille B (2001) Ion channels of excitable membranes. Sinauer Associates, Sunderland
Hinsch KD, De Pinto V, Aires VA, Schneider X, Messina A, Hinsch E (2004) Voltage-dependent anion-selective channels VDAC2 and VDAC3 are abundant proteins in bovine outer dense fibers, a cytoskeletal component of the sperm flagellum. J Biol Chem 279:15281–15288
Hogg RC, Wang Q, Large WA (1994) Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol 112:977–984
Hu JH, He XB, Wu Q, Yan YC, Koide SS (2002) Biphasic effect of GABA on rat sperm acrosome reaction: involvement of GABA(A) and GABA(B) receptors. Arch Androl 48:369–378
Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY (2009) Studies on expression and function of the TMEM16A calcium-activated chloride channels. Proc Natl Acad Sci USA 106(50):21413–21418
Hung PH, Suarez SS (2010) Regulation of sperm storage and movement in the ruminant oviduct. Soc Reprod Fertil Suppl 67:257–266
Ingermann RL, Holcomb M, Zuccarelli MD, Kanuga MK, Cloud JG (2008) Initiation of motility by steelhead (Oncorhynchus mykiss) sperm: membrane ion exchangers and pH sensitivity. Comp Biochem Physiol A Mol Integr Physiol 151:651–656
Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R (2011) Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci USA 108:20008–20011
Jentsch TJ, Neagoe I, Scheel O (2005) CLC chloride channels and transporters. Curr Opin Neurobiol 15:319–325
Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ (2006) Calcium signalling in human spermatozoa: a specialized ‘toolkit’ of channels, transporters and stores. Hum Reprod Update 12:253–267
Jimenez-Gonzalez MC, Gu Y, Kirkman-Brown J, Barratt CL, Publicover S (2007) Patch-clamp ‘mapping’ of ion channel activity in human sperm reveals regionalisation and co-localisation into mixed clusters. J Cell Physiol 213:801–808
Jin JY, Chen WY, Zhou CX, Chen ZH, Yu-Ying Y, Ni Y, Chan HC, Shi QX (2009) Activation of GABAA receptor/Cl− channel and capacitation in rat spermatozoa: HCO3 − and Cl− are essential. Syst Biol Reprod Med 55:97–108
Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N (2011) Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA 108:4892–4896
Jung J, Nam JH, Park HW, Oh U, Yoon JH, Lee MG (2013) Dynamic modulation of ANO1/TMEM16A HCO3(−) permeability by Ca2+/calmodulin. Proc Natl Acad Sci USA 110:360–365
Kanbara K, Mori Y, Kubota T, Watanabe M, Yanagawa Y, Otsuki Y (2011) Expression of the GABAA receptor/chloride channel in murine spermatogenic cells. Histol Histopathol 26:95–106
Kaupp UB, Kashikar ND, Weyand I (2008) Mechanisms of sperm chemotaxis. Annu Rev Physiol 70:93–117
Kirichok Y, Lishko PV (2011) Rediscovering sperm ion channels with the patch-clamp technique. Mol Hum Reprod 17:478–499
Kirichok Y, Navarro B, Clapham DE (2006) Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature (Lond) 439:737–740
Konig J, Schreiber R, Voelcker T, Mall M, Kunzelmann K (2001) The cystic fibrosis transmembrane conductance regulator (CFTR) inhibits ENaC through an increase in the intracellular Cl− concentration. EMBO Rep 2:1047–1051
Kumar P, Meizel S (2008) Identification and spatial distribution of glycine receptor subunits in human sperm. Reproduction 136:387–390
Kunzelmann K, Kongsuphol P, Chootip K, Toledo C, Martins JR, Almaca J, Tian Y, Witzgall R, Ousingsawat J, Schreiber R (2011) Role of the Ca2+-activated Cl− channels bestrophin and anoctamin in epithelial cells. Biol Chem 392:125–134
Kunzelmann K, Schreiber R (1999) CFTR, a regulator of channels. J Membr Biol 168:1–8
Kwon WS, Park YJ, Mohamed-el SA, Pang MG (2013) Voltage-dependent anion channels are a key factor of male fertility. Fertil Steril 99:354–361
Labarca P, Santi C, Zapata O, Morales E, Beltr'an C, Li'evano A, Darszon A (1996) A cAMP regulated K+-selective channel from the sea urchin sperm plasma membrane. Dev Biol 174:271–280
Li CY, Jiang LY, Chen WY, Li K, Sheng HQ, Ni Y, Lu JX, Xu WX, Zhang SY, Shi QX (2010) CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum Reprod 25:317–327
Lievano A, Sanchez JA, Darszon A (1985) Single-channel activity of bilayers derived from sea urchin sperm plasma membranes at the tip of a patch-clamp electrode. Dev Biol 112:253–257
Lievano A, Santi CM, Serrano CJ, Trevino CL, Bellve AR, Hernandez-Cruz A, Darszon A (1996) T-type Ca2+ channels and alpha1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett 388:150–154
Lindemann CB, Goltz JS (1988) Calcium regulation of flagellar curvature and swimming pattern in triton X-100–extracted rat sperm. Cell Motil Cytoskeleton 10:420–431
Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE (2012) The control of male fertility by spermatozoan ion channels. Annu Rev Physiol 74:453–475
Liu B, Wang Z, Zhang W, Wang X (2009) Expression and localization of voltage-dependent anion channels (VDAC) in human spermatozoa. Biochem Biophys Res Commun 378:366–370
Liu B, Zhang W, Wang Z (2010) Voltage-dependent anion channel in mammalian spermatozoa. Biochem Biophys Res Commun 397:633–636
Llanos MN, Ronco AM, Aguirre MC, Meizel S (2001) Hamster sperm glycine receptor: evidence for its presence and involvement in the acrosome reaction. Mol Reprod Dev 58:205–215
Macdonald RL, Olsen RW (1994) GABAA receptor channels. Annu Rev Neurosci 17:569–602
Martinez-Lopez P, Santi CM, Trevino CL, Ocampo-Gutierrez AY, Acevedo JJ, Alisio A, Salkoff LB, Darszon A (2009) Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem Biophys Res Commun 381:204–209
Martinez-Lopez P, Trevino CL, de la Vega-Beltran JL, Blas GD, Monroy E, Beltran C, Orta G, Gibbs GM, O’Bryan MK, Darszon A (2011) TRPM8 in mouse sperm detects temperature changes and may influence the acrosome reaction. J Cell Physiol 226(6):1620–1631
Mayorga LS, Tomes CN, Belmonte SA (2007) Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life 59:286–292
McPartlin LA, Visconti PE, Bedford-Guaus SJ (2011) Guanine-nucleotide exchange factors (RAPGEF3/RAPGEF4) induce sperm membrane depolarization and acrosomal exocytosis in capacitated stallion sperm. Biol Reprod 85:179–188
Medina JF, Recalde S, Prieto J, Lecanda J, Saez E, Funk CD, Vecino P, van Roon MA, Ottenhoff R, Bosma PJ, Bakker CT, Elferink RP (2003) Anion exchanger 2 is essential for spermiogenesis in mice. Proc Natl Acad Sci USA 100:15847–15852
Meizel S (1997) Amino acid neurotransmitter receptor/chloride channels of mammalian sperm and the acrosome reaction. Biol Reprod 56:569–574
Meizel S, Son JH (2005) Studies of sperm from mutant mice suggesting that two neurotransmitter receptors are important to the zona pellucida-initiated acrosome reaction. Mol Reprod Dev 72:250–258
Meizel S, Turner KO (1996) Chloride efflux during the progesterone-initiated human sperm acrosome reaction is inhibited by lavendustin A, a tyrosine kinase inhibitor. J Androl 17:327–330
Melendrez CS, Meizel S (1995) Studies of porcine and human sperm suggesting a role for a sperm glycine receptor/Cl− channel in the zona pellucida-initiated acrosome reaction. Biol Reprod 53:676–683
Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA (2004) Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA 101:16501–16506
Miledi R (1982) A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci 215:491–497
Morales E, de la Torre L, Moy GW, Vacquier VD, Darszon A (1993) Anion channels in the sea urchin sperm plasma membrane. Mol Reprod Dev 36:174–182
Moreno C, Vaca L (2011) SOC and now also SIC: store-operated and store-inhibited channels. IUBMB Life 63:856–863
Munoz-Garay C, De la Vega-Beltran JL, Delgado R, Labarca P, Felix R, Darszon A (2001) Inwardly rectifying K(+) channels in spermatogenic cells: functional expression and implication in sperm capacitation. Dev Biol 234:261–274
Navarro B, Kirichok Y, Clapham DE (2007) KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA 104:7688–7692
Navarro B, Miki K, Clapham DE (2011) ATP-activated P2X2 current in mouse spermatozoa. Proc Natl Acad Sci USA 108:14342–14347
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS (2004) Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA 101:13483–13488
Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y (1985) Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem 260:9699–9705
Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE (2004) Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279:33742–33750
Orta G, Ferreira G, Jose O, Trevino CL, Beltran C, Darszon A (2012) Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction. J Physiol (Lond) 590:2659–2675
Ottolia M, Toro L (1994) Potentiation of large conductance K/Ca channels by niflumic, flufenamic, and mefenamic acids. Biophys J 67:2272–2279
Pace AJ, Lee E, Athirakul K, Coffman TM, O’Brien DA, Koller BH (2000) Failure of spermatogenesis in mouse lines deficient in the Na(+)-K(+)-2Cl(-) cotransporter. J Clin Invest 105:441–450
Peixoto PM, Dejean LM, Kinnally KW (2012) The therapeutic potential of mitochondrial channels in cancer, ischemia-reperfusion injury, and neurodegeneration. Mitochondrion 12:14–23
Perez-Cornejo P, Arreola J (2004) Regulation of Ca(2+)-activated chloride channels by cAMP and CFTR in parotid acinar cells. Biochem Biophys Res Commun 316:612–617
Petit FM, Serres C, Bourgeon F, Pineau C, Auer J (2013) Identification of sperm head proteins involved in zona pellucida binding. Hum Reprod 28:852–865
Pifferi S, Dibattista M, Menini A (2009) TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Arch 458:1023–1038
Popli K, Stewart J (2007) Infertility and its management in men with cystic fibrosis: review of literature and clinical practices in the UK. Hum Fertil (Camb) 10:217–221
Publicover S, Harper CV, Barratt C (2007) [Ca2+] i signalling in sperm: making the most of what you’ve got. Nat Cell Biol 9:235–242
Publicover SJ, Barratt CL (2012) Chloride channels join the sperm ‘channelome’. J Physiol (Lond) 590:2553–2554
Puente MA, Tartaglione CM, Ritta MN (2011) Bull sperm acrosome reaction induced by gamma-aminobutyric acid (GABA) is mediated by GABAergic receptors type A. Anim Reprod Sci 127:31–37
Quill TA, Ren D, Clapham DE, Garbers DL (2001) A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA 98:12527–12531
Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE (2001) A sperm ion channel required for sperm motility and male fertility. Nature (Lond) 413:603–609
Ren D, Xia J (2010) Calcium signaling through CatSper channels in mammalian fertilization. Physiology (Bethesda) 25:165–175
Ritta MN, Bas DE, Tartaglione CM (2004) In vitro effect of gamma-aminobutyric acid on bovine spermatozoa capacitation. Mol Reprod Dev 67:478–486
Ritta MN, Calamera JC, Bas DE (1998) Occurrence of GABA and GABA receptors in human spermatozoa. Mol Hum Reprod 4:769–773
Rossato M, Di Virgilio F, Rizzuto R, Galeazzi C, Foresta C (2001) Intracellular calcium store depletion and acrosome reaction in human spermatozoa: role of calcium and plasma membrane potential. Mol Hum Reprod 7:119–128
Russell JM (2000) Sodium-potassium-chloride cotransport. Physiol Rev 80:211–276
Santi CM, Darszon A, Hernandez-Cruz A (1996) A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am J Physiol 271:C1583–C1593
Santi CM, Martinez-Lopez P, de la Vega-Beltran JL, Butler A, Alisio A, Darszon A, Salkoff L (2010) The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 584:1041–1046
Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF (2003) Cell volume regulation and swelling-activated chloride channels. Biochim Biophys Acta 1618:153–162
Sato Y, Son JH, Meizel S (2000) The mouse sperm glycine receptor/chloride channel: cellular localization and involvement in the acrosome reaction initiated by glycine. J Androl 21:99–106
Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L (1998) Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem 273:3509–3516
Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134:1019–1029
Scudieri P, Sondo E, Ferrera L, Galietta LJ (2012) The anoctamin family: TMEM16A and TMEM16B as calcium-activated chloride channels. Exp Physiol 97:177–183
Sheppard DN, Welsh MJ (1999) Structure and function of the CFTR chloride channel. Physiol Rev 79:S23–S45
Shi QX, Roldan ER (1995) Evidence that a GABAA-like receptor is involved in progesterone-induced acrosomal exocytosis in mouse spermatozoa. Biol Reprod 52:373–381
Shi QX, Yuan YY, Roldan ER (1997) Gamma-aminobutyric acid (GABA) induces the acrosome reaction in human spermatozoa. Mol Hum Reprod 3:677–683
Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N (2010) VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 31:227–285
Sieghart W (1995) Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev 47:181–234
Sigel E, Steinmann ME (2012) Structure, function, and modulation of GABA(A) receptors. J Biol Chem 287:40224–40231
Sones WR, Leblanc N, Greenwood IA (2009) Inhibition of vascular calcium-gated chloride currents by blockers of KCa1.1, but not by modulators of KCa2.1 or KCa2.3 channels. Br J Pharmacol 158:521–531
Stamboulian S, Kim D, Shin HS, Ronjat M, De Waard M, Arnoult C (2004) Biophysical and pharmacological characterization of spermatogenic T-type calcium current in mice lacking the CaV3.1 (alpha1G) calcium channel: CaV3.2 (alpha1H) is the main functional calcium channel in wild-type spermatogenic cells. J Cell Physiol 200:116–124
Suarez SS (2008) Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 52:455–462
Suarez SS, Varosi SM, Dai X (1993) Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci USA 90:4660–4664
Tian Y, Schreiber R, Kunzelmann K (2012) Anoctamins are a family of Ca2+-activated Cl− channels. J Cell Sci 125:4991–4998
Toma C, Greenwood IA, Helliwell RM, Large WA (1996) Activation of potassium currents by inhibitors of calcium-activated chloride conductance in rabbit portal vein smooth muscle cells. Br J Pharmacol 118:513–520
Travis AJ, Kopf GS (2002) The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest 110:731–736
Trevino CL, Felix R, Castellano LE, Gutierrez C, Rodriguez D, Pacheco J, Lopez-Gonzalez I, Gomora JC, Tsutsumi V, Hernandez-Cruz A, Fiordelisio T, Scaling AL, Darszon A (2004) Expression and differential cell distribution of low-threshold Ca(2+) channels in mammalian male germ cells and sperm. FEBS Lett 563:87–92
Triphan X, Menzel VA, Petrunkina AM, Cassara MC, Wemheuer W, Hinsch KD, Hinsch E (2008) Localisation and function of voltage-dependent anion channels (VDAC) in bovine spermatozoa. Pflugers Arch 455:677–686
Turner KO, Garcia MA, Meizel S (1994) Progesterone initiation of the human sperm acrosome reaction: the obligatory increase in intracellular calcium is independent of the chloride requirement. Mol Cell Endocrinol 101:221–225
Turner TT (2008) De Graaf's thread: the human epididymis. J Androl 29:237–250
Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS (1995a) Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development (Camb) 121:1129–1137
Visconti PE, Florman HM (2010) Mechanisms of sperm–egg interactions: between sugars and broken bonds. Sci Signal 3:pe35
Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS (1999) Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem 274:3235–3242
Visconti PE, Krapf D, de la Vega-Beltran JL, Acevedo JJ, Darszon A (2011) Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl 13:395–405
Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS (1995b) Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development (Camb) 121:1139–1150
Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, Garbers DL (2007) A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc Natl Acad Sci USA 104:9325–9330
Wang J, Haanes KA, Novak I (2013) Purinergic regulation of CFTR and Ca2+-activated Cl− channels and K+ channels in human pancreatic duct epithelium. Am J Physiol Cell Physiol 304(7):C673–C684
Webb TI, Lynch JW (2007) Molecular pharmacology of the glycine receptor chloride channel. Curr Pharm Des 13:2350–2367
Wennemuth G, Carlson AE, Harper AJ, Babcock DF (2003) Bicarbonate actions on flagellar and Ca2+ -channel responses: initial events in sperm activation. Development (Camb) 130:1317–1326
Wertheimer EV, Salicioni AM, Liu W, Trevino CL, Chavez J, Hernandez-Gonzalez EO, Darszon A, Visconti PE (2008) Chloride is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J Biol Chem 283:35539–35550
Weyand I, Godde M, Frings S, Weiner J, Muller F, Altenhofen W, Hatt H, Kaupp UB (1994) Cloning and functional expression of a cyclic-nucleotide-gated channel from mammalian sperm. Nature (Lond) 368:859–863
Wistrom CA, Meizel S (1993) Evidence suggesting involvement of a unique human sperm steroid receptor/Cl− channel complex in the progesterone-initiated acrosome reaction. Dev Biol 159:679–690
Wood CD, Darszon A, Whitaker M (2003) Speract induces calcium oscillations in the sperm tail. J Cell Biol 161:89–101
Wood CD, Nishigaki T, Tatsu Y, Yumoto N, Baba SA, Whitaker M, Darszon A (2007) Altering the speract-induced ion permeability changes that generate flagellar Ca2+ spikes regulates their kinetics and sea urchin sperm motility. Dev Biol 306:525–537
Wu WL, So SC, Sun YP, Chung YW, Grima J, Wong PY, Yan YC, Chan HC (1998) Functional expression of P2U receptors in rat spermatogenic cells: dual modulation of a Ca(2+)-activated K+ channel. Biochem Biophys Res Commun 248:728–732
Xia J, Ren D (2009) The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod Biol Endocrinol 7:119
Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M (2006) Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 296:353–362
Xu HM, Li HG, Xu LG, Zhang JR, Chen WY, Shi QX (2012) The decline of fertility in male uremic patients is correlated with low expression of the cystic fibrosis transmembrane conductance regulator protein (CFTR) in human sperm. Hum Reprod 27:340–348
Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi Liu G, Zhu H, Ma ZG, Wang XF, Chen ZH, Zhou SC, Dong HS, Zhang XH, Chung YW, Yuan YY, Yang WX, Chan HC (2007) Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci USA 104:9816–9821
Yanagimachi R (1994) Mammalian fertilization. In: Knobile E, Neill JD (eds) The physiology of reproduction. Raven, New York, pp 189–317
Yanagimachi R (1998) Intracytoplasmic sperm injection experiments using the mouse as a model. Hum Reprod 13(Suppl 1):87–98
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature (Lond) 455:1210–1215
Yeung CH, Barfield JP, Cooper TG (2005) Chloride channels in physiological volume regulation of human spermatozoa. Biol Reprod 73:1057–1063
Yeung CH, Barfield JP, Cooper TG (2006) Physiological volume regulation by spermatozoa. Mol Cell Endocrinol 250:98–105
Yeung CH, Cooper TG (2008) Potassium channels involved in human sperm volume regulation–quantitative studies at the protein and mRNA levels. Mol Reprod Dev 75:659–668
Zanetti N, Mayorga LS (2009) Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod 81:396–405
Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM (2011) Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci USA 108:5879–5884
Zeng Y, Clark EN, Florman HM (1995) Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev Biol 171:554–563
Acknowledgments
This work was supported by DGAPA: IN202312 (to A.D.) and IN202212-3 (to C.T.), CONACyT: 128566 (to A.D.) and 99333 (to C.T.), CSIC-UdelaR international collaboration grants to G.F. with A.D. and C.T. NIH: R01 HD038082 (to Pablo Visconti) and CSIC p944 (to Gonzalo Ferreira).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Treviño, C.L. et al. (2014). Cl− Channels and Transporters in Sperm Physiology. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)