Abstract
Allurin, a 21-kDa protein secreted by the oviduct of female Xenopus frogs, is incorporated into the jelly layers of eggs as they pass single file on their way to the uterus and subsequent spawning. Hydration of the egg jelly layers at spawning releases allurin as a chemoattractant that binds to the midpiece of Xenopus sperm in a dose-dependent manner. Gradients of allurin elicit directed swimming across a porous membrane in two-chamber assays and preferential, up-gradient swimming of sperm in video-microscopic assays. Allurin, purified from X. laevis or produced in recombinant form, also elicits chemotaxis by mouse sperm in two-chamber and video microscopic assays. Allurin binds to mouse sperm at the midpiece and head, a pattern also seen in frog sperm. Western blots suggest the presence of an allurin-like protein in the follicular fluid of mice and humans and peptides that mimic subdomains within allurin elicit chemoattractive behavior in both mouse and human sperm. By sequence homology, allurin is a truncated member of the Cysteine-RIch Secretory Protein (CRISP) family whose members include Crisps 1, 2, and 4, which have been demonstrated to modulate mammalian sperm functions including capacitation, ion channel activity, and sperm–egg binding. Interestingly, allurin contains only two of the three domains found in these full-length CRISP proteins and in this respect is similar to the sperm self-recognition proteins HrUrabin and CiUrabin important in ascidian gamete interactions. These findings suggest that both full-length and truncated CRISP proteins play important reproductive roles in species widely separated in evolutionary time.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Sperm physiological responses are choreographed by a series of signaling ligands generated by female gametes and the organs in which they are housed. The signals conveyed inform the sperm of the location, direction, and status of nearby female gametes and elicit changes in sperm motility, sperm secretory status, and sperm metabolic activities required to successfully fertilize an egg. Some of these ligands are diffusible in nature and emanate from extracellular coatings surrounding the egg whereas other ligands form a more stable part of the extracellular coat structure and act only on sperm in contact with these matrices. Diffusible ligands include peptides, steroids, amino acids, and even proteins (Burnett et al. 2008a).
One class of protein ligands that have received increasing interest are the cysteine-rich secretory (Crisp) proteins whose members include snake venom toxins, sperm chemoattractants, sperm decapacitation factors, sperm self-identification markers, and sperm ion channel modulators (Burnett et al. 2008a; Gibbs et al. 2008; Kratzschmar et al. 1996; Koppers et al. 2011). Full-length Crisp proteins contain three domains, as indicated in Fig. 4.1. The largest “pathogenesis-related” (PR) domain at the N-terminal (cyan) is homologous to proteins expressed in a variety of plants in response to environmental stress or viral infection (Fernandez et al. 1997). Next is the Hinge domain (yellow), the smallest of the three, named for the fact that it links the first and third domain. Last is the ion channel regulatory (ICR) domain at the C-terminal (magenta), named for its ability to block or modulate a variety of voltage-dependent potassium and calcium channels as well as ryanodine receptors (Gibbs et al. 2006). The ICR domain is similar in tertiary structure to ion channel-blocking peptides from sea anenomes (Pennington et al. 1999; Alessandri-Haber et al. 1999; Cotton et al. 1997).
The mammalian Crisp proteins, 1 through 4, are reproductively important and full length. Earliest reports of sperm-associated Crisp proteins demonstrated that the rodent epididymis secretes Crisp 1, which binds to the post-acrosomal region of the sperm head (Cohen et al. 2011). This protein has been shown to migrate to the equatorial region of rat sperm during capacitation and to possibly participate in sperm–egg fusion (Cohen et al. 2000, 2007, 2008; Da Ros et al. 2004; Roberts et al. 2006, 2007, 2008; Ellerman et al. 2006). Antibodies raised against the protein inhibit sperm–egg fusion, and the protein has been demonstrated to bind to the egg surface (Cohen et al. 2007; Ellerman et al. 2006). Although knockout of the protein does not produce sterility, it does reduce the efficiency of sperm–egg binding in vitro (Da Ros et al. 2008).
A second Crisp family member, TPX-1 or Crisp 2, discovered as an acrosomal granule constituent and as a major autoantigen in vasectomized rodents (Hardy et al. 1988; Foster and Gerton 1996), has also been shown to play a modulatory role in sperm–egg binding in rodents (Busso et al. 2007). Crisp 2 is thought to be released during the acrosome reaction, and the protein has been shown to help mediate binding of the sperm to the zona pellucida (Cohen et al. 2011).
Crisp 3, although widely expressed, is a prominent secreted protein in the prostate (Udby et al. 2005). This protein can be found in the circulation bound to a macroglobulin and has been proposed for use as a marker of prostatic hypertrophy (Bjartell et al. 2006; Udby et al. 2010). As a constituent of semen, the protein has been correlated with reproductive success in horses and cows (Schambony et al. 1998a, b; Topfer-Petersen et al. 2005).
Crisp 4, present in rodents but not in other mammals, has been the first Crisp family protein demonstrated to have a molecular role in sperm physiology. Darszon and coworkers (Gibbs et al. 2010a) have shown that Crisp 4 inhibits the opening of TRP-M8 channels in mouse sperm that are required to initiate capacitation. Channel inhibition by Crisp 4 or possibly by its homologue Crisp 1 may play a role in preventing premature capacitation, that is, acting as a “decapacitation factor.” In addition, Crisp 4 has been shown to modulate sperm binding to the zona pellucida (Turunen et al. 2012). Found in the epididymis, this protein is considered to be a functional orthologue to human Crisp 1 (Nolan et al. 2006).
The complexity of the CRISP family has been further increased by the discovery of “truncated” members missing the “ion channel regulatory” domain characteristic of full-length Crisp proteins such as Crisp 1 and Crisp 4. As shown in Fig. 4.1, truncated members typically contain the PR and Hinge domains along with amino-acid sequences at the C-terminal that vary in length from a few residues to several hundred. Members of this class include allurin, the frog sperm chemoattractant whose characteristics are described in the current chapter, insect venom and hookworm antigen proteins (Burnett et al. 2008a), the protease inhibitor-like proteins P115 and P116 (Gibbs et al. 2008), Ciona intestinalis (Ci) Urabin and Halocynthia roretzi (Hr) Urabin, recently discovered self-identity ligands on ascidian sperm (Urayama et al. 2008; Yamaguchi et al. 2011), and the glioma pathogenesis related-like proteins, whose functions are currently unknown, but which include members that are expressed in a testis-specific manner (Gibbs et al. 2010b).
Commonly, all these proteins are grouped into the Cysteine Rich–Antigen–Pathogenesis related (CAP) superfamily of proteins that are characterized by a homologous PR domain, with four regions of particularly high sequence homology referred to as “CAP signatures” or “CRISP signatures” (green in Fig. 4.1) (Gibbs et al. 2008).
2 Characterization of Allurin as a Frog Sperm Chemoattractant
Amphibian eggs undergo a marked decrease in fertilizability if their outer jelly layers are removed. This observation was initially made in Bufo japonicas and Bufo arenaras and more recently in Xenopus laevis (Katagiri 1987; Krapf et al. 2009; Olson and Chandler 1999). However, reintroduction of diffusible jelly components (referred to as “egg water”) restores the fertilizability of these jellyless eggs almost to their original level of 80 % or greater (Olson and Chandler 1999). For this reason, our laboratory tested the possibility that a diffusible jelly component from X. laevis eggs might be acting as a sperm chemoattractant by using two types of assays (Burnett et al. 2011a). The first uses a modified Boyden chamber that has a porous polycarbonate filter separating an upper sperm chamber and a lower chemoattractant chamber. Sperm passing through the filter in response to a chemotactic gradient of egg water were increased fivefold over those in control experiments in which egg water was not present (Al-Anzi and Chandler 1998). This response was dose dependent, egg water specific, and was not seen in the presence of egg water mixed uniformly throughout the lower chamber. This assay guided the subsequent purification of allurin from egg water using anion-exchange chromatography in conjunction with a NaCl step gradient for elution (Olson et al. 2001; Sugiyama et al. 2009).
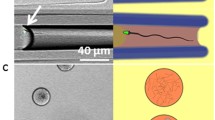
A second chemoattraction assay, developed by Sally Zigmond to study neutrophils (Zigmond 1977) and modified by Giojalas and coworkers for use with sperm (Fabro et al. 2002), employs video microscopy to record the directional movements of individual sperm. Trajectories are recorded of sperm swimming on an observational platform that lies between two troughs, one acting as a reservoir of sperm and the other as a reservoir of chemoattractant (see Fig. 4.2a). The sperm on the platform swim within a chemotactic gradient formed by diffusion. Their movement at 7 frame/s was analyzed for velocity and directionality using X–Y Cartesian coordinates, the gradient axis being along the X coordinate. Values in successive frames as well as average values over entire trajectories were compared.
Swimming behavior of Xenopus laevis sperm in a gradient of egg water that contains allurin. (a) Cut-away diagram of an inverted Zigmond chamber. Sperm trajectories are tracked under the observation platform of the chamber by video microscopy. The chamber is inverted because these sperm cannot swim against gravity. Inset: Sperm in the observation area are exposed to a gradient of chemoattractant. (b) Sperm velocity is unchanged in the presence of an egg-water gradient (red/dark bars) regardless of whether the sperm a swimming up or down the gradient. (c) The average net travel of sperm along the gradient (X) axis is increased threefold in the presence of an egg-water gradient but is not increased along the Y axis. (d) Distribution of net travel along the gradient (X) axis for individual sperm is shifted to large positive values in the presence of an egg-water gradient (red/dark bars) providing evidence of directed sperm movement toward the chemoattractant trough. Error bars represent the mean ± SE of 150 sperm in three experiments. (a reproduced with permission from Burnett et al. 2012; b–d reproduced from Burnett et al. 2011c)
Data analysis revealed that in the presence of an egg-water gradient (red/dark bars, Fig. 4.2b) average sperm velocity was about 43 μm/s and not significantly different from sperm velocity in the absence of a gradient (white bars, Fig. 4.2b). In addition, similar sperm velocities were observed in sperm traveling up the gradient and down the gradient, suggesting that egg water does not contain a chemokinetic agent that alters sperm swimming speed. In contrast, net sperm travel along the X coordinate (gradient axis) was increased threefold by the presence of an egg-water gradient (first bar set, Fig. 4.2c). In comparison, sperm movement along the Y coordinate perpendicular to the gradient axis was random and not increased by egg water (second bar set, Fig. 4.2c). Sperm movement along the X coordinate, when analyzed in greater detail, showed a marked change in trajectory distribution from relatively random movement (white bars, Fig. 4.2d) to those favoring positive, up-gradient movement (red/dark bars, Fig. 4.2d). These findings demonstrated that X. laevis (Xl) sperm prefer to swim toward the reservoir of egg water, indicating the presence of a chemoattractant.
3 Allurin Is a Chemoattractant for Mammalian Sperm
Because rodents express a number of Crisp proteins that have striking roles in sperm physiology and sperm–egg interactions, it was of interest to determine whether a truncated Crisp protein such as allurin had effects on mammalian sperm. Initially we used the two-chamber assay to demonstrate that a gradient of X. laevis egg water did indeed have the ability increase mouse sperm passage across a porous membrane by 2.5 fold (Burnett et al. 2011b, 2012). Subsequently, we tested the ability of mouse sperm to bind Alexa 488-conjugated allurin in a region-specific manner. Dye-conjugated allurin bound strongly to the subequatorial region of the mouse sperm head and in a punctuate pattern to the midpiece of the sperm flagellum (see Fig. 4.3c, d). Remarkably, this pattern of binding was similar to that observed when frog sperm were exposed to the same allurin conjugate (Fig. 4.3a, b).
Fluorescence micrographs of Oregon green 488-conjugated allurin bound to frog and mouse sperm. (a, b) Allurin binds to X. laevis sperm at the midpiece and to a variable extent at the head. No binding is observed at the flagellum. Bars = 2 μm. (c, d) Allurin binds to mouse sperm at the subequatorial region of the head (arrows) and at the midpiece of the flagellum (dashed lines). Bars = 10 μm. (a and b reproduced with permission from Burnett et al. 2011c; c and d reproduced with permission from Burnett et al. 2011b)
These findings suggested the need for a more detailed study of allurin-induced chemotaxis in mouse sperm using the Zigmond chamber. In these studies we compared the effects of allurin purified from egg water with those of mouse follicular fluid, a known source of chemoattractants for mouse sperm. Mouse sperm, in contrast to Xl sperm, are strong swimmers and can swim against gravity. Thus, the observational platform of the Zigmond chamber can face upward when evaluating mouse sperm (Fig. 4.4a, inset), whereas the observational platform faces downward when observing Xl sperm (Fig. 4.2a, inset). As shown in Fig. 4.4b, both purified allurin (red/dark bar) and mouse follicular fluid (yellow/light bar) gradients produced no marked change in sperm swimming velocity when compared to sperm swimming in the absence of a gradient (white bar, Fig. 4.4b). In contrast, the net distance swum by sperm along the X (gradient) axis was increased dramatically in the presence of either an allurin or a follicular fluid gradient (first bar set, Fig. 4.4c). Such an increase was not seen along the Y-axis but instead random movement having a net displacement near zero, much like controls in which no chemotactic agent is present (second bar set, Fig. 4.4c). Differences in movement along the gradient (X)-axis between chemotaxing sperm and control sperm are clearly seen in distribution plots such as that in Fig. 4.4d. The presence of either an allurin (red/dark bars) or follicular fluid (yellow/light bars) gradient markedly decreases the percentage of sperm swimming down the gradient while markedly increasing the percentage of sperm swimming up gradient toward the chemoattractant trough.
Swimming behavior of mouse sperm in allurin and follicular fluid gradients. (a) Sperm movement is tracked on the observation platform of a Zigmond chamber. Inset: Sperm in the observation area are exposed to a gradient of chemoattractant emanating from the trough. (b) Average forward velocity of sperm in an allurin gradient (red/dark bar) and in a mouse follicular fluid gradient (yellow/light bar) is similar to that in controls (open bar). (c) The average net travel of sperm along the gradient (X) axis is substantial in the presence of an allurin or a follicular fluid gradient. (d) Distribution of differences in sperm travel along the gradient (X) axis (presence of chemoattractant - absence). Data is binned according to magnitude of X axis travel (vertical line is zero). The percentage of sperm traveling down gradient away from the chemoattractant was markedly reduced while the percentage of sperm traveling up gradient was markedly increased. Error bars represent the mean ± SE for 80 sperm in four experiments. AL allurin, MFF mouse follicular fluid. (a reproduced with permission from Burnett et al. 2012; b, c, d reproduced from Burnett et al. 2011b)
These data provide strong evidence that allurin binds to and produces sperm chemoattractant behavior in both Xenopus and mouse sperm. Given that the identity of sperm chemoattractants in follicular fluid is still a matter of debate, these data also provide impetus for asking whether there are allurin-like chemoattractant proteins in follicular fluid. Two observations suggest that further study is warranted. First, immunocytochemistry using anti-allurin antibodies strongly labels the mural granulosa and cumulus cells surrounding the oocyte in secondary ovarian follicles of the mouse (Fig. 4.5). The localization appears to be cytoplasmic and is consistent with granulosa cell secretion of an allurin-like protein. Second, the secretion of such a protein into the follicular fluid can be verified by Western blot. As shown in Fig. 4.6, mouse follicular fluid, although containing hundreds of proteins of varying molecular weights (left, FF, Commassie stained), reveals just one band at 20 kDa that labels with anti-allurin antibodies (right, FF, double asterisk). This finding suggests the presence of a truncated Crisp protein in follicular fluid, albeit one whose identity and chemoattractant activity is presently unknown.
Fluorescence immunocytochemical staining of a secondary follicle in the mouse ovary using anti-allurin antibodies. A strong signal is seen in the cytoplasm of mural granulosa cells and in the cumulus cells surrounding the oocyte. The antibodies used cross-react with full-length Crisp proteins, thus demonstrating their expression in developing follicles. Draq 5 was used as a nuclear counterstain. Bar = 25 μm. (Reproduced with permission from Burnett et al. 2012)
Western blot detection of an allurin-like protein in mouse follicular fluid. Follicular fluid (FF) contains numerous Coomassie blue-stained proteins spanning a wide range of sizes as shown by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (left, FF). Western blotting of the gel with anti-allurin antibodies reveals one labeled band at a relative mobility of 20 kDa (right, FF, double asterisk). This band is similar in mobility to the monomer band in purified X. laevis allurin (right, AL, single asterisk). (Reproduced with permission from Burnett et al. 2011b)
4 The Future of Crisp Protein Relationships in Reproduction
In retrospect, the discovery that allurin is also a chemoattractant for mammalian sperm should not have been surprising. CAP superfamily and Crisp subfamily proteins have entered into a broad array of reproductive mechanisms across both vertebrate and invertebrate animal phyla. Evolutionary relationships can be brought out by comparing domain organization and the positioning of the highly conserved cysteines which, through formation of disulfide bonds, play a vital role in tertiary structure and stability.
The PR domain, found as a stand-alone domain in both plants and animals, may have been the earliest domain of the CAP superfamily to evolve. It presumed origin before to the plant–animal bifurcation is hinted at by the existence of current-era yeast proteins that exhibit substantial homology to this domain Choudhary and Schneiter 2012). This domain, consisting of a β-pleated sheet sandwiched between α-helices on each face, is stabilized by three disulfide bonds (Fernandez et al. 1997). The PR domain has taken on a number of suspected roles during evolution including cation binding and proteolytic and disintegrin activities, as well as abilities to bind to sperm and oocyte surfaces.
Metal binding most notably includes high-affinity coordination with cadmium and zinc, abilities that could be related to sequestering of potentially lethal metals in the case of cadmium or a basis for metalloprotease activity in the case of zinc. The coordinating residues for cadmium or zinc binding have been clearly predicted from the X-ray crystallographic structures of Crisp snake toxins (Wang et al. 2005; Guo et al. 2005; Shikamoto et al. 2005). Similarly, the structure of the Crisp-related protein glioma pathogenesis-related protein 1 in its zinc bound form has been determined (Asojo et al. 2011). Indeed, the PR domain structure bears some similarity to Zn2+-requiring metalloproteases although only a few Crisp-related proteins have proven activity (e.g., Milne et al. 2003). However, these structural similarities may underlie the fact that the PR domain is capable of docking with a number of protein partners in other contexts.
A more detailed inspection of the sequence of this domain in a variety of CAP/CRISP proteins shows the presence of a PR domain (blue shading, Fig. 4.7) having at least four CAP/CRISP signature regions of high amino-acid identity (green shading, Fig. 4.7) and six to eight cysteines that are generally disulfide bonded (red on yellow, Fig. 4.7). Four of these cysteines [residues 73, 148, 153, and 164 in Xenopus tropicalis (Xt) allurin] are essentially invariant. In contrast, the positioning of two other cysteines shows variation between two subgroups of Crisp proteins. One subgroup represented by mammalian Crisps, snake toxin crisps, and a lamprey Crisp protein have these cysteines within the CAP/CRISP 2 and -4 signature sequences (residues 93 and 167 in Xt allurin). A second subgroup represented by plant PR-1 and ascidian sperm self-recognition proteins have these two cysteines near the CAP/CRISP signature 1 sequence at residues 109 and 115 in the PR-1 protein of tomato (arrows, Fig. 4.7). Xt allurin and a hypothetical Crisp protein in finch both conform perfectly to the first subgroup and thus might be expected to have a tertiary structure much like that delineated in the X-ray diffraction structures of snake toxin Crisp proteins. If so, one can expect all six of the cysteines in the PR domains of these proteins to be disulfide bonded and the pattern of bonding to be overlapping as in the toxin proteins (Wang et al. 2005; Guo et al. 2005).
Clustal W-aligned amino-acid sequences of homologous CAP/CRISP proteins. Signal sequences and initial nonhomologous N-terminal regions have been omitted for clarity. The PR, Hinge, and ICR (ion channel regulatory) domains are shaded in blue, yellow, and pink respectively. The PR domain includes four CAP/CRISP signature sequences of high homology (boxed and shaded in green). Cysteines are highlighted in red on yellow. Initial residue positions are indicated at the left of each sequence line. Double slashes indicate short internal sequences omitted for clarity. Genbank acquisition numbers for the sequences are: AJ011520.1/GI:3660528, M98858.1/GI:162550, AF393653.2/GI:317185854, NM001201342.1/GI:318065112, XM002191406.1/GI:224048894, AY288089.1/GI:31075034, NM001246286.1/GI:350534677, BAG68488.1/GI:195972735, NP689992.1/GI:22749527, BC011150.1/GI:15029853, AY324325.1/GI:32492058, BAF56484.1/GI:145046200, AJ491318.1/GI:32187774
Unexpectedly, some truncated Crisp proteins, including X. laevis (Xl) allurin and wasp venom proteins, do not conform to either pattern of cysteines and in fact have been shown to have two free cysteines that are not disulfide bonded (Sugiyama et al. 2009; Henriksen et al. 2001). What this means for the tertiary structure of Xl allurin is not clear, although its structure in the PR domain is likely to be quite different from that of Xt allurin. It comes as a surprise then that Xl allurin and Xt allurin show similar chemoattractant activity, even across species (Burnett et al. 2008b); this would seem to imply that the chemoattractant activity of the allurins is mediated by a different region of the PR domain or by the hinge domain as hypothesized next.
Indeed, the PR domain appears to have come into greater use when coupled with the smaller hinge domain. Although the origin of the hinge domain is uncertain, its amino-acid sequence is highly homologous in all CAP/CRISP proteins and notably features four invariant cysteines (yellow/light shading, Fig. 4.7). In X-ray diffraction structures of CAP proteins these cysteines form a pair of overlapping disulfide bonds that maintain the consistent “chair-like” tertiary structure of this domain (Wang et al. 2005; Guo et al. 2005). The widespread use of this PR domain–Hinge domain combination in Crisp proteins ranges from ascidians, worms, and snails in the invertebrates to amphibians, snakes, and mammals in the vertebrates. Given this, it may pertinent to ask what kinds of increased functionality have been gained by the use of this domain together with the PR domain.
One possibility is that rather than acting independently, these two domains might both contribute surfaces that together allow new protein–protein interactions. An interesting piece of data that hints at this comes from the X-ray crystal structure of pseudecin, one of the Crisp protein snake toxins from the viper Pseudecis porphyriacus that inhibits cyclic nucleotide gated channels (Suzuki et al. 2008). In this protein, the surface formed by the C-terminal-most region of the PR domain and a surface formed by the hinge domain face one another to provide a deep cleft that could be a site of protein docking. Indeed, binding of Zn2+ by this protein results in a marked shift in the positions of these two surfaces, and the authors have postulated that this may regulate the interaction of this protein with the channel (Suzuki et al. 2008). Similar tantalizing results come from our studies of peptides representing sequences within allurin that mimic this region of the PR and Hinge domains. Synthetic peptides representing the Hinge region and the C-terminal of the PR domain have been found to have dose-dependent chemoattractant activity for both frog and mouse sperm in two-chamber assays (Washburn et al. 2011; Washburn, unpublished observations). In contrast, peptides of random sequence or having homologous sequences from snake toxin Crisp proteins do not show activity. Of further interest is that these peptides bind to sperm at the midpiece, the same spatial pattern seen in binding of allurin to sperm.
As indicated in Fig. 4.1, full-length Crisp proteins contain the PR domain–Hinge domain combination just discussed as well as the ion channel regulatory (ICR) domain. The ICR domain, as previously mentioned, has been demonstrated to modulate the actions of voltage-dependent potassium channels, voltage-dependent calcium channels, and ryanodine receptors (Gibbs et al. 2006; Brown et al. 1999; Morrissette et al. 1995; Nobile et al. 1994, 1996). These properties target channels in muscle and nerve during the actions of Crisp protein toxins found in snakes and lizards. Although the presence of Crisp proteins in insect venoms would seem to be a parallel use it should be pointed out that the ICR domain is not present in the truncated Crisp proteins found in these venoms.
In mammals, full-length Crisp proteins are associated with sperm production in the testis, sperm maturation in the epididymis, and sperm capacitation and oocyte binding in the female reproductive tract (Gibbs et al. 2008; Burnett et al. 2008a). One might speculate that the ICR domain of these proteins may be modifying the behavior of ion channels in sperm. Ion channel activity is important in sperm capacitation, and there is evidence that Crisp 1 may be acting as a decapacitation factor that keeps certain channels quiescent in the male tract, but which upon removal during capacitation in the female tract, allows new channel activity (Nixon et al. 2006). Indeed, Crisp 4, a rodent orthologue of Crisp 1, has been shown to modify TRP-M8 channels in mouse sperm that may be involved in sperm capacitation and chemotaxis (Gibbs et al. 2010a).
Interestingly, the ICR domain has structural similarities to sea anemome toxin peptides that have been shown to block voltage-dependent potassium and calcium channels. In vertebrates, however, the ICR does not appear as an independent domain but is virtually always within the context of a full-length Crisp protein. This observation would seem to imply that the PR domain–Hinge domain combination associated with it must play some role in enhancing the actions of the ICR domain. One possibility is that these domains are important for cell-surface binding and localization so as to target ICR domain actions. Evidence for this capacity comes from the fact that Crisp 1 binds to the mouse sperm surface in the epididymis and remains bound to the sperm in the female tract (Cohen et al. 2000; Da Ros et al. 2004; Roberts et al. 2002, 2008). Its localization on the sperm and its binding lifetime are dependent on its glycosylation state. The “D” form of Crisp 1 binds to the sperm head in the postacrosomal region and remains bound until ejaculated into the female tract. The “D” form inhibits premature acrosome reaction in the male tract, but once in the female tract appears to unbind from the sperm during capacitation (Roberts et al. 2008; Nixon et al. 2006). In contrast, the “E” form of Crisp 1, having a different glycosylation pattern, binds to sperm in the epididymis, but is not easily removed even in the female tract (Da Ros et al. 2004; Roberts et al. 2008); rather, it migrates to the equatorial region of the sperm where it is thought to take part in sperm binding at the egg plasma membrane (Cohen et al. 2008, 2011).
Indeed, binding of Crisp 1 and Crisp 2 to the egg plasma membrane of rats has been characterized (Ellerman et al. 2006; Cohen et al. 2011). The structural feature of Crisp 1 responsible for egg binding appears to lie in the CAP/CRISP 2 signature region of the PR domain. This signature is common to both Crisp 1 and Crisp 2, and the fact that these two Crisp proteins compete for the same site on the egg surface suggests that this signature is likely the binding partner in both proteins (Cohen et al. 2011). If peptides representing this signature are incubated with eggs, peptide binding is not only detected but is accompanied by block of sperm–egg plasma membrane adhesion and subsequent fertilization (Ellerman et al. 2006). Similarly, we have shown that allurin, absent an ICR domain, binds to the sperm head at the equatorial region in a manner similar to Crisp 1, but different in binding to the midpiece of the flagellum as well (see Fig. 4.3).
Of additional interest is that the “E” form of Crisp 1, during production in the epididymis, is proteolytically processed in a manner that eliminates the ICR domain, leaving a truncated Crisp 1 that bears a lot of similarity to allurin, albeit highly glycosylated (Roberts et al. 2002). This point raises the possibility that proteolytic processing of a full-length Crisp protein in the ovarian follicle could lead to the allurin-like protein detected in mouse follicular fluid that we have hypothesized might bind to sperm and act as a chemoattractant (see Fig. 4.7).
Although the binding partners of mammalian Crisp proteins on the egg surface are not yet known, binding partners of Crisp proteins have been studied in sperm. In mouse sperm, a cell-surface protein designated SHTAP has been shown to bind Crisp 2, be expressed exclusively in the testis, and to relocalize during sperm capacitation (Jamsai et al. 2009). In addition, binding of Crisp 2 to SHTAP in a yeast two-hybrid system seems to require both the PR and Hinge domains as well as the ICR domain to maximize binding. Again, the region in the PR domain required for binding was that of the CAP/CRISP 2 signature sequence at the C-terminal of the domain (Jamsai et al. 2009).
Parallel data come from truncated Crisp proteins found on the surface of ascidian sperm. As demonstrated by Sawada and coworkers, ascidian sperm display GPI-anchored Crisp proteins that play a role in mediating self-recognition between sperm and egg so as to prevent self-fertilization (Urayama et al. 2008; Yamaguchi et al. 2011). In two different species, H. roretzi and C. intestinalis, a GPI-anchored Urabin on the surface of the sperm head binds to a specific partner protein in the vitelline coat of the conspecific egg (VC70 and VC57 in the two respective species). Initial binding of the sperm Urabin to the vitelline coat supports subsequent binding of a pair of polycystin-1-like proteins on the sperm surface (s-Themis A and B) with binding partners on the vitelline coat (v-Themis A and B) (Yamaguchi et al. 2011). Both these specific sperm–vitelline coat interactions are then thought to lead to weakening of the interaction and removal of the sperm, thus blocking fertilization.
In summary, data from multiple sperm species support the hypothesis that the CAP/CRISP signatures 1 and 2 mediate binding and localization of Crisp proteins to target cells and this binding is enhanced by the Hinge domain. In some cases, as in the mammalian and snake venom Crisp proteins, such binding may be a requirement for actions of the ICR domain on ion channels in the membrane of the target cell. In the case of allurin this binding appears to be to a yet-uncharacterized receptor leading to signal transduction events that modulate sperm flagellar activity and chemotaxis.
5 Conclusion
Allurin, a 21-kDa protein synthesized in the Xenopus oviduct, is incorporated into the jelly surrounding spawned Xenopus eggs. Upon release into the surrounding medium the protein binds to an uncharacterized receptor on the Xenopus sperm midpiece and elicits chemotactic behavior; that is, sperm preferentially swim up an allurin gradient toward higher concentrations of the protein. Surprisingly, allurin elicits a similar response in mouse sperm. The amino acid sequence of allurin indicates that it is a truncated member of the Crisp family having two domains. Our results suggest that either the pathogenesis-related domain or Hinge domain of allurin activates an evolutionarily conserved signaling system that controls flagellar waveform and directional swimming. Future research is needed to determine whether Crisp protein signaling systems play a role in mammalian sperm chemotaxis in vivo.
References
Al-Anzi B, Chandler DE (1998) A sperm chemoattractant is released from Xenopus egg jelly during spawning. Dev Biol 198:366–375
Alessandri-Haber N, Lecoq A, Gasparini S et al (1999) Mapping the functional anatomy of BgK on Kv1.1, Kv1.2, and Kv1.3. Clues to design analogs with enhanced selectivity. J Biol Chem 274:35653–35661
Asojo OA, Koski RA, Bonafé N (2011) Structural studies of human glioma pathogenesis-related protein 1. Acta Crystallogr D Biol Crystallogr 67:847–855
Bjartell A, Johansson R, Bjork T et al (2006) Immunohistochemical detection of cysteine-rich secretory protein 3 in tissue and in serum from men with cancer or benign enlargement of the prostate gland. Prostate 66:591–603
Brown RL, Haley TL, West KA et al (1999) Pseudechetoxin: a peptide blocker of cyclic nucleotide-gated ion channels. Proc Natl Acad Sci USA 96:754–759
Burnett LA, Xiang X, Bieber AL et al (2008a) Crisp proteins and sperm chemotaxis: discovery in amphibians and explorations in mammals. Int J Dev Biol 52:489–501
Burnett LA, Boyles S, Spencer C et al (2008b) Xenopus tropicalis allurin: expression, purification and characterization of a sperm chemoattractant that exhibits cross-species activity. Dev Biol 316:408–416
Burnett LA, Tholl N, Chandler DE (2011a) Two types of assays for detecting frog sperm chemoattraction. J Vis Exp 58(1-8):e3407. doi:10.3791/3407
Burnett L, Anderson D, Rawls A et al (2011b) Mouse sperm exhibit chemotaxis to allurin, a truncated member of the cysteine-rich secretory protein family. Dev Biol 360:318–328
Burnett L, Sugiyama H, Bieber A et al (2011c) Egg jelly proteins stimulate directed motility in Xenopus laevis sperm. Mol Reprod Dev 78:450–462
Burnett LA, Washburn CA, Sugiyama H et al (2012) Allurin, an amphibian sperm chemoattractant having implications for mammalian sperm physiology. Int Rev Cell Mol Biol 295:1–61
Busso D, Goldweic N, Hayashi M et al (2007) Evidence for the involvement of testicular protein CRISP2 in mouse sperm–egg fusion. Biol Reprod 76:701–708
Choudhary V, Schneiter R (2012) Pathogen-related yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proc Natl Acad Sci USA 109:16882–16887
Cohen DJ, Rochwerger L, Ellerman DA et al (2000) Relationship between the association of rat epididymal protein “DE” with spermatozoa and the behavior and function of the protein. Mol Reprod Dev 56:180–188
Cohen DJ, Da Ros VG, Busso D et al (2007) Participation of epididymal cysteine-rich secretory proteins in sperm–egg fusion and their potential use for male fertility regulation. Asian J Androl 9:528–532
Cohen DJ, Busso D, Da Ros V et al (2008) Participation of cysteine-rich secretory proteins (CRISP) in mammalian sperm–egg interaction. Int J Dev Biol 52:737–742
Cohen DJ, Maldera JA, Vasen G et al (2011) Epididymal protein CRISP1 plays different roles during the fertilization process. J Androl 32:672–678
Cotton J, Crest M, Couet F et al (1997) A potassium-channel toxin from the sea anemone Bunodosoma granulifera, an inhibitor for Kv1 channels. Revision of the amino acid sequence, disulfide-bridge assignment, chemical synthesis, and biological activity. Eur J Biochem 244:192–202
Da Ros VG, Munice MJ, Cohen DJ et al (2004) Bicarbonate is required for migration of sperm epididymal protein DE (CRISP 1) to the equatorial segment and expression of rat sperm fusion ability. Biol Reprod 70:1325–1332
Da Ros VG, Maldera JA, Willis WD et al (2008) Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev Biol 320:12–18
Ellerman DA, Cohen DJ, Da Ros VG et al (2006) Sperm protein “DE” mediates gamete fusion through an evolutionarily conserved site of the CRISP family. Dev Biol 297:228–237
Fabro G, Rovasio RA, Civalero S et al (2002) Chemotaxis of capacitated rabbit spermatozoa to follicular fluid revealed by a novel directionality-based assay. Biol Reprod 67:1565–1571
Fernandez C, Szyperski T, Bruyere T et al (1997) NMR solution structure of the pathogenesis-related protein P14a. J Mol Biol 266:576–593
Foster GA, Gerton JA (1996) Autoantigen 1 of the guinea pig sperm acrosome is the homologue of mouse Tpx-1 and human TPX1 and is a member of the cysteine-rich secretory protein (CRISP) family. Mol Reprod Dev 44:221–229
Gibbs GM, Scanlon MJ, Swarbrick J et al (2006) The cysteine-rich secretory protein domain of Tpx-1 is related to ion channel toxins and regulates ryanodine receptor calcium signaling. J Biol Chem 281:4156–4163
Gibbs GM, Roelants K, O’Bryan MK (2008) The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins–roles in reproduction, cancer, and immune defense. Endocr Rev 29:865–897
Gibbs GM, Orta G, Reddy T et al (2010a) Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc Natl Acad Sci USA 108:7034–7039
Gibbs GM, Lo JCY, Nixon B et al (2010b) Glioma pathogenesis-related 1-like 1 is enriched, dynamically modified, and redistributed during male germ cell maturation and has a potential role in sperm–oocyte binding. Endocrinology 151:2331–2342
Guo M, Teng M, Niu L et al (2005) Crystal structure of the cysteine-rich secretory protein stecrisp reveals that the cysteine-rich domain has a K+ channel inhibitor-like fold. J Biol Chem 280:12405–12412
Hardy DM, Huang TT, Driscoll WJ et al (1988) Purification and characterization of the primary acrosomal autoantigen of guinea pig epididymal spermatozoa. Biol Reprod 38:423–437
Henriksen A, King TP, Mirza O et al (2001) Major venom allergen of yellow jackets, Ves v 5: structural characterization of a pathogenesis-related protein superfamily. Proteins 45:438–448
Jamsai D, Rijal S, Bianco DM et al (2009) A novel protein, sperm head and tail associated protein (SHTAP), interacts with cysteine-rich secretory protein 2 (CRISP2) during spermatogenesis in the mouse. Biol Cell 102:93–106
Katagiri C (1987) Role of oviductal secretions in mediating gamete fusion in anuran amphibians. Zool Sci 4:1–14
Koppers AJ, Reddy T, O’Bryan MK (2011) The role of cysteine-rich secretory proteins in male fertility. Asian J Androl 13:111–117
Krapf D, O’Brien ED, Cabada MO et al (2009) Egg water from the amphibian Bufo arenarum modulates the ability of homologous sperm to undergo the acrosome reaction in the presence of the vitelline envelope. Biol Reprod 80:311–319
Kratzschmar J, Haendler B, Eberspaecher U et al (1996) The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP 1, CRISP 2 and CRISP 3. Eur J Biochem 236:827–836
Milne TJ, Abbenante G, Tyndall JD et al (2003) Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J Biol Chem 278:31105–31110
Morrissette J, Kratzschmar J, Haendler B et al (1995) Primary structure and properties of helothermine, a peptide toxin that blocks ryanodine receptors. Biophys J 68:2280–2288
Nixon B, MacIntyre DA, Mitchell LA et al (2006) The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol Reprod 74:275–287
Nobile M, Magnelli V, Lagostena L et al (1994) The toxin helothermine affects potassium currents in newborn rat cerebellar granule cells. J Membr Biol 139:49–55
Nobile M, Noceti F, Prestipino G et al (1996) Helothermine, a lizard venom toxin, inhibits calcium current in cerebellar granules. Exp Brain Res 110:15–20
Nolan MA, Wu L, Bang HJ et al (2006) Identification of rat cysteine-rich secretory protein 4 (Crisp 4) as the ortholog to human CRISP 1 and mouse Crisp 4. Biol Reprod 74:984–991
Olson JH, Chandler DE (1999) Xenopus laevis egg jelly contains small proteins that are essential for fertilization. Dev Biol 210:401–410
Olson J, Xiang X, Ziegert T et al (2001) Allurin, a 21 kD sperm chemoattractant from Xenopus egg jelly, is homologous to mammalian sperm-binding proteins. Proc Natl Acad Sci USA 98:11205–11210
Pennington MW, Lanigan MD, Kalman K et al (1999) Role of disulfide bonds in the structure and potassium channel blocking activity of ShK toxin. Biochemistry 38:14549–14558
Roberts KP, Ensrud KM, Hamilton DW (2002) A comparative analysis of expression and processing of the rat epididymal fluid and sperm-bound forms of proteins D and E. Biol Reprod 67:525–533
Roberts KP, Ensrud KM, Wooters JL et al (2006) Epididymal secreted protein Crisp 1 and sperm function. Mol Cell Endocrinol 250:122–127
Roberts KP, Johnston D, Nolan MA et al (2007) Structure and function of epididymal protein cysteine-rich secretory protein-1. Asian J Androl 9:508–514
Roberts KP, Ensrud-Bowlin KM, Piehl LB et al (2008) Association of the protein D and protein E forms of rat CRISP1 with epididymal sperm. Biol Reprod 79:1046–1053
Schambony A, Hess O, Gentzel M et al (1998a) Expression of CRISP proteins in the male equine genital tract. J Reprod Fertil Suppl 53:67–72
Schambony A, Gentzel M, Wolfes H et al (1998b) Equine CRISP 3: primary structure and expression in the male genital tract. Biochim Biophys Acta 1387:206–216
Shikamoto Y, Suto K, Yamazaki Y et al (2005) Crystal structure of a CRISP family Ca2+-channel blocker derived from snake venom. J Mol Biol 350:735–743
Sugiyama H, Burnett L, Xiang X et al (2009) Purification and multimer formation of allurin, a sperm chemoattractant from Xenopus laevis egg jelly. Mol Reprod Dev 76:527–536
Suzuki N, Yamazaki Y, Brown RL et al (2008) Structures of pseudechetoxin and pseudecin, two snake-venom cysteine-rich secretory proteins that target cyclic nucleotide-gated ion channels: implications for movement of the C-terminal cysteine-rich domain. Acta Crystallogr D 64:1034–1042
Topfer-Petersen E, Ekhlasi-Hundrieser M, Kirchhoff C et al (2005) The role of stallion seminal proteins in fertilisation. Anim Reprod Sci 89:159–170
Turunen H, Sipila P, Krutskikh A et al (2012) Loss of cysteine-rich secretory protein 4 (Crisp4) leads to deficiency in sperm–zona pellucida interaction in mice. Biol Reprod 86:121–128
Udby L, Bjartell A, Malam J et al (2005) Characterization and localization of cysteine-rich secretory protein 3 (CRISP 3) in the human male reproductive tract. J Androl 26:333–342
Udby L, Johnsen AH, Borregaard N (2010) Human CRISP-3 binds serum alpha(1)B-glycoprotein across species. Biochim Biophys Acta 1800:481–485
Urayama S, Harada Y, Nakagawa Y et al (2008) Ascidian sperm glycosylphosphatidylinositol-anchored CRISP-like protein as a binding partner for an allorecognizable sperm receptor on the vitelline coat. J Biol Chem 283:21725–21733
Wang J, Shen B, Guo M et al (2005) Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry 44:10145–10152
Washburn CA, Bieber AL, Tubbs K et al (2011) Mammalian sperm chemotaxis is elicited by peptide mimics of cysteine rich secretory proteins. In: Abstracts, National meeting of the Society for the Study of Reproduction, Portland
Yamaguchi A, Saito T, Yamada L et al (2011) Identification and localization of the sperm CRISP family protein CiUrabin involved in gamete interaction in the ascidian Ciona intestinalis. Mol Reprod Dev 78:488–497
Zigmond SH (1977) Ability of polymorphonulear leukocytes to orient in gradients of chemical factors. J Cell Biol 75:606–616
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
This chapter is dedicated to Allan L. Bieber, a long-time collaborator of ours who recently passed on. Allan was an expert biochemist who guided our purification of allurin and characterization of its disulfide bonding pattern using mass spectrometry. His long-term interest in venom proteins from snakes was culminated by his delight in finding that allurin is closely related to Crisp snake toxin proteins.
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Burnett, L., Sugiyama, H., Washburn, C., Bieber, A., Chandler, D.E. (2014). Allurin: Exploring the Activity of a Frog Sperm Chemoattractant in Mammals. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_4
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)