Abstract
The molecular basis of gamete recognition and the corresponding block to polyspermy in mammals have intrigued investigators for decades. Taking advantage of the fastidious nature of human sperm , which will not bind to the mouse zona pellucida , gain-of-function assays have been established in transgenic mice by replacing endogenous mouse proteins with the corresponding human homologue. In the presence of human ZP2 , by itself or with the three other human zona proteins, human sperm bind and penetrate the ‘humanized’ zona pellucida but do not fuse with the mouse egg. Using recombinant ZP2 peptides in a bead binding assay, the gamete recognition site was located to a ~115 amino-acid N-terminal domain. Following fertilization , egg cortical granules exocytose ovastacin , an oocyte-specific metalloendoprotease , that cleaves the N-terminus of ZP2 and prevents sperm binding to the zona surrounding the preimplantation embryo. Genetic ablation of the enzyme or mutation of the ZP2 cleavage site prevents cleavage and sperm bind de novo to the surface of the zona pellucida even after fertilization and cortical granule exocytosis. These observations form the basis of the ZP2 cleavage model of gamete recognition in which mammalian sperm bind to an N-terminal domain of ZP2. Following penetration through the zona matrix and gamete fusion, the egg cortical granules exocytose ovastacin, which cleaves ZP2 and provides a definitive block to sperm binding at the surface of the zona pellucida.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The fertilizing sperm is capacitated by passage through the female reproductive tract. As sperm approach ovulated eggs in the ampulla of the oviduct , the acrosome , a subcellular organelle underlying the sperm surface, remains intact. The ovulated egg(s) is surrounded by two investments: a gelatinous cumulus mass composed of hyaluronan interspersed with cumulus cells and an insoluble, extracellular zona pellucida . The successful sperm navigates the cumulus mass, and binds and penetrates the zona pellucida before entry into the perivitelline space between the zona matrix and egg. Only acrosome-reacted sperm are observed in the perivitelline space, but the site and molecular basis of induction of acrosome exocytosis remain controversial and the function(s) of acrosomal contents remains an area of active investigation (Avella and Dean 2011). Acrosome-reacted sperm fuse with the egg plasma membrane and fertilization activates the egg.
The zona pellucida is an extracellular matrix surrounding eggs and early embryos that plays critical roles in gamete recognition required for fertilization and in ensuring monospermy necessary for the successful onset of development (Fig. 33.1a). The mouse and human zona pellucida are composed of three and four (ZP1 , ZP2 , ZP3 , ZP4 ) glycoproteins, respectively (Bleil and Wassarman 1980; Shabanowitz and O’Rand 1988; Lefievre et al. 2004). Although each zona protein is well conserved (62–71 % amino-acid identity), human sperm are fastidious and will not bind to the mouse zona pellucida (Bedford 1977). Following fertilization, the zona pellucida is modified and mouse sperm do not bind to two-cell embryos. Although a number of changes in the zona matrix have been inferred, only biochemical cleavage of ZP2 has been experimentally documented (Bleil et al. 1981; Bauskin et al. 1999). The zona pellucida also plays a critical role in ensuring passage of the early embryo through the oviduct before implantation. The biochemical removal of the zona matrix leads to resorption of the embryo into the epithelia lining of the oviduct and is lethal (Bronson and McLaren 1970; Modlinski 1970).
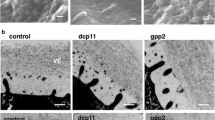
Role of the zona pellucida in fertilization and early development. (a) Ovulated eggs in the cumulus mass are fertilized by a single sperm in the ampulla of the oviduct . Each haploid gamete forms a pronucleus and after syngamy develops into a one-cell zygote that divides within 24 h to form the two-cell embryo. The extracellular zona pellucida that surrounds the egg is permissive for sperm binding and penetration (green). However, following fertilization the zona matrix is modified (red) and sperm do not bind. The zona pellucida is critically important for gamete recognition , a postfertilization block to sperm binding, and for protecting the embryo as it passes through the oviduct. (Modified from Li et al. 2013). (b) An insoluble, extracellular zona pellucida (~7 μm wide) surrounds mouse eggs (~80 μm diameter) (Familiari et al. 2008). Mouse sperm are ~125 μm long with a thin acrosome overlying a distinctive falciform (hook-like) head; the zona matrix has multiple pores that may facilitate sperm penetration. (c) The human zona pellucida (~15 μm) surrounds a larger egg (~120 μm) and has a structure in scanning electron microscopy (EM) similar to that of mouse (Familiari et al. 2006). Human sperm are half as long (~ 60 μm) as mouse sperm with a smaller, flattened, spatulate head
Using mouse genetics, we have investigated the molecular basis of gamete recognition , the postfertilization block to polyspermy , and the role of the zona in protecting the early embryo. Based on experimental results, we propose a ZP2 cleavage model of gamete recognition in which sperm bind to the N-terminus of ZP2, which is cleaved by ovastacin to prevent postfertilization sperm binding (Gahlay et al. 2010; Baibakov et al. 2012; Burkart et al. 2012). The absence of ZP2 or ZP3 precludes formation of a zona pellucida surrounding ovulated eggs and leads to resorption and embryonic lethality (Liu et al. 1996; Rankin et al. 1996; Rankin et al. 2001).
2 The Structure of the Zona Pellucida
By scanning electron microscopy, the mouse and human zonae pellucidae appear similar, with fenestration thought to provide passage for sperm penetration (Familiari et al. 2006; Familiari et al. 2008). The mouse egg and surrounding zona pellucida are smaller than those of the human, although the mouse sperm is considerably longer than the diminutive human sperm (Fig. 33.1b,c). As noted, the human zona pellucida has four glycoproteins, but the mouse zona pellucida has only three. Each zona pellucida protein is encoded by a single-copy gene that is found on syntenic chromosomes in mouse and human (Hoodbhoy et al. 2005). However, mouse Zp4 is an expressed pseudogene that is not translated into protein because of multiple missense and stop codons (Lefièvre et al. 2004).
Each of the other three mouse zona genes has been ablated in embryonic stem cells to establish mouse lines lacking the cognate protein. Zp1 null mice form a zona pellucida with a more loosely woven matrix than normal. Female mice are fertile, albeit with decreased fecundity (Rankin et al. 1999). However, mice lacking either ZP2 (Rankin et al. 2001) or ZP3 (Rankin et al. 1996; Liu et al. 1996) do not form a zona pellucida surrounding ovulated eggs , and female mice are sterile. Thus, ZP1 is not essential for fertility, but in the absence of a zona matrix, these loss-of-function observations do not provide insight into the role of either ZP2 or ZP3 in gamete recognition .
3 ZP2 Cleavage Model of Gamete Recognition
In more recent genetic studies, we have taken advantage of two physiological dichotomies. The first is that human sperm are fastidious and bind to the human, but not the mouse, zona pellucida. The second is that homologous mouse sperm bind to eggs but not two-cell embryos . To exploit the first dichotomy, we have used mouse genetics to replace each endogenous mouse protein with the corresponding human protein; we have established transgenic mice expressing human ZP4 ; and we have crossed the four lines together to establish the quadruple rescue line that expresses the four human zona proteins and none of the three mouse zona proteins (Rankin et al. 1998; Rankin et al. 2003; Yauger et al. 2011; Baibakov et al. 2012).
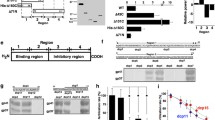
Each of these lines formed a zona pellucida and was fertile when mated with normal male mice. Eggs from each line were tested for their ability to support capacitated human sperm binding in vitro using eggs in cumulus (Fig. 33.2a). Only a zona matrix with human ZP2 , either by itself or in conjunction with the three other human zona proteins was able to support human sperm binding. In the presence of all four human proteins, sperm penetrated the zona matrix and accumulated in the perivitelline space , unable to fuse with mouse eggs. Following fertilization with mouse sperm , an effective postfertilization block to polyspermy was established and human sperm were unable to bind to the zona pellucida. The binding site on ZP2 was further defined by assaying sperm binding to beads coated with recombinant human and mouse N-terminal ZP2 peptides. Human sperm bound to beads coated with human ZP239-154, but not to the corresponding mouse ZP2 peptide, and binding was dependent on structural constraints imposed by disulfide bonds (Baibakov et al. 2012).
Human and mouse sperm binding to genetically altered zonae pellucidae. (a) Confocal and differential interference contrast (DIC) images of capacitated human sperm binding to the zona pellucida of mice expressing human ZP1 , human ZP2 , human ZP3 , or human ZP4 , in the absence of the corresponding mouse protein. Human sperm bind only to human ZP2 in transgenic mice. (Modified from Baibakov et al. 2012). (b) Capacitated mouse sperm binding to two-cell embryos in which ZP2 remains intact because ZP2 was mutated to prevent postfertilization cleavage (Zp2 Mut). Zp2 Mut eggs serve as positive controls and normal two-cell embryos as negative controls. (Modified from Gahlay et al. 2010)
Following fertilization , ZP2 is cleaved and the cleavage site was defined by microscale Edman degradation as immediately upstream of a diacidic motif, a known recognition site for the astacin family of metalloendoproteases. When ZP2 was mutated in transgenic mice to prevent postfertilization cleavage (166LA↓DE169 → 166LG↓AA169), mouse sperm bound de novo to two-cell embryos despite fertilization and cortical granule exocytosis (Fig. 33.2b) (Gahlay et al. 2010). Ovastacin is an oocyte-specific metalloendoprotease that is conserved in mouse and human (Quesada et al. 2004). Using a peptide-specific antibody, mouse ovastacin was detected in cortical granules of normal eggs and was absent in two-cell embryos, consistent with its postfertilization exocytosis. The single-copy gene Astl encodes ovastacin. When Astl was ablated in transgenic mice, homozygous null female were fertile with a modest decrease in fecundity. However, ZP2 remained uncleaved following fertilization and mouse sperm bound to the zona pellucida surrounding two-cell embryos (Burkart et al. 2012).
Cortical granule exocytosis is associated with a postfertilization block to polyspermy that occurs via multiple mechanisms and is critically important to ensure monospermy essential for normal development. The most rapid block prevents supernumerary sperm already present in the perivitelline space from fusing with the egg plasma membrane. Although attributed to membrane depolarization in other species, the molecular basis of this block remains to be determined in mammals (Jaffe et al. 1983; Horvath et al. 1993). There is an additional block to penetration of the zona pellucida that appears to occur within the first hour after fertilization (Sato 1979; Stewart-Savage and Bavister 1988). During the ensuing several hours, ovastacin cleaves ZP2 after which sperm do not bind to the zona pellucida (Baibakov et al. 2007). Although slow compared to the first two blocks and probably of less immediate importance, cleavage of ZP2 provides a definitive and irreversible block to polyspermy.
These observations are consistent with earlier observations in Xenopus laevis in which gp69/64 , the homologue of mouse ZP2 , inhibited primary sperm binding to eggs in vitro (Tian et al. 1997). Following fertilization , gp69/64 was cleavage at the conserved 155FD↓DD158 site and the C-terminal native gp69/64 glycopeptide did not inhibit sperm binding. Although a short recombinant peptide gp69/64130-156 also did not affect sperm binding, the longer gp69/6434-156 N-terminal domain (homologous to mouse ZP235-149) was not tested. Following fertilization, pg69/64 is cleaved by a zinc metalloprotease (Lindsay and Hedrick 2004), and postfertilization proteolysis of the N-terminal domain of gp69/64 could account for the lack of sperm binding in Xenopus embryos . Thus, the ZP2 cleavage model of gamete recognition may apply broadly among vertebrates.
4 The Model and Future Validation
The ZP2 cleavage model provides a simple, unifying explanation to account for these experimental observations in transgenic mice (Fig. 33.3). To wit, capacitated sperm bind to the N-terminus of ZP2 in the extracellular zona pellucida surrounding ovulated eggs . After penetration of the zona matrix, the gamete membranes are fused at fertilization . Postfertilization cortical granule exocytosis releases ovastacin , which diffuses through the zona matrix. This metalloendoprotease cleaves mouse ZP2 at 166LA↓DE169 and provides a definitive block to polyspermy . The zona pellucida that surrounds the early embryo is essential to ensure passage through the oviduct for implantation and the successful onset of development.
The ZP2 cleavage model of gamete recognition . (a) The mouse zona pellucida is composed of three glycoproteins, ZP1 , ZP2, and ZP3 . Capacitated sperm bind to an N-terminal domain of ZP2, penetrate through the zona matrix, and fertilize the egg by fusing with its membrane: this activates the egg and leads to cortical granule exocytosis, which releases ovastacin , a metalloendoprotease . Ovastacin cleaves ZP2 within the zona pellucida and provides a definitive block to sperm binding in the early embryo. (b) Same as a, but with the human zona pellucida composed of four glycoproteins
This model makes predictions that can be tested experimentally with transgenic mice expressing chimeric human–mouse or truncated forms of ZP2 . If the model remains robust, a more precise definition of the sperm -binding site may provide reagents to identify the long elusive cognate sperm receptor . Particularly satisfying would be the replacement of the mouse sperm receptor with the human homologue, after which ‘humanized’ sperm should not bind to the mouse zona pellucida unless it contains human ZP2.
References
Avella MA, Dean J (2011) Fertilization with acrosome-reacted mouse sperm: implications for the site of exocytosis. Proc Natl Acad Sci USA 108:19843–19844
Baibakov B, Gauthier L, Talbot P et al (2007) Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development (Camb) 134:933–943
Baibakov B, Boggs NA, Yauger B et al (2012) Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J Cell Biol 197:897–905
Bauskin AR, Franken DR, Eberspaecher U et al (1999) Characterization of human zona pellucida glycoproteins. Mol Hum Reprod 5:534–540
Bedford JM (1977) Sperm/egg interaction: the specificity of human spermatozoa. Anat Rec 188:477–488
Bleil JD, Wassarman PM (1980) Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte’s zona pellucida. Dev Biol 76:185–202
Bleil JD, Beall CF, Wassarman PM (1981) Mammalian sperm–egg interaction: fertilization of mouse eggs triggers modification of the major zona pellucida glycoprotein, ZP2. Dev Biol 86:189–197
Bronson RA, McLaren A (1970) Transfer to the mouse oviduct of eggs with and without the zona pellucida. J Reprod Fertil 22:129–137
Burkart AD, Xiong B, Baibakov B et al (2012) Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J Cell Biol 197:37–44
Familiari G, Heyn R, Relucenti M et al (2006) Ultrastructural dynamics of human reproduction, from ovulation to fertilization and early embryo development. Int Rev Cytol 249:53–141
Familiari G, Heyn R, Relucenti M et al (2008) Structural changes of the zona pellucida during fertilization and embryo development. Front Biosci 13:6730–6751
Gahlay G, Gauthier L, Baibakov B et al (2010) Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science 329:216–219
Hoodbhoy T, Joshi S, Boja ES et al (2005) Human sperm do not bind to rat zonae pellucidae despite the presence of four homologous glycoproteins. J Biol Chem 280:12721–12731
Horvath PM, Kellom T, Caulfield J et al (1993) Mechanistic studies of the plasma membrane block to polyspermy in mouse eggs. Mol Reprod Dev 34:65–72
Jaffe LA, Sharp AP, Wolf DP (1983) Absence of an electrical polyspermy block in the mouse. Dev Biol 96:317–323
Lefievre L, Conner S, Salpekar A et al (2004) Four zona pellucida glycoproteins are expressed in the human. Hum Reprod 19:1580–1586
Lefièvre L, Conner SJ, Salpekar A et al (2004) Four zona pellucida glycoproteins are expressed in the human. Hum Reprod 19:1580–1586
Li L, Lu X, Dean J (2013) The maternal to zygotic transition in mammals. Mol Aspects Med. doi:10.1016/j.mam.2013.01.003
Lindsay LL, Hedrick JL (2004) Proteolysis of Xenopus laevis egg envelope ZPA triggers envelope hardening. Biochem Biophys Res Commun 324:648–654
Liu C, Litscher ES, Mortillo S et al (1996) Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci USA 93:5431–5436
Modlinski JA (1970) The role of the zona pellucida in the development of mouse eggs in vivo. J Embryol Exp Morphol 23:539–547
Quesada V, Sanchez LM, Alvarez J et al (2004) Identification and characterization of human and mouse ovastacin: a novel metalloproteinase similar to hatching enzymes from arthropods, birds, amphibians, and fish. J Biol Chem 279:26627–26634
Rankin T, Familari M, Lee E et al (1996) Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development (Camb) 122:2903–2910
Rankin TL, Tong Z-B, Castle PE et al (1998) Human ZP3 restores fertility in Zp3 null mice without affecting order-specific sperm binding. Development (Camb) 125:2415–2424
Rankin T, Talbot P, Lee E et al (1999) Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development (Camb) 126:3847–3855
Rankin TL, O’Brien M, Lee E et al (2001) Defective zonae pellucidae in Zp2 null mice disrupt folliculogenesis, fertility and development. Development (Camb) 128:1119–1126
Rankin TL, Coleman JS, Epifano O et al (2003) Fertility and taxon-specific sperm binding persist after replacement of mouse ‘sperm receptors’ with human homologues. Dev Cell 5:33–43
Sato K (1979) Polyspermy-preventing mechanisms in mouse eggs fertilized in vitro. J Exp Zool 210:353–359
Shabanowitz RB, O’Rand MG (1988) Characterization of the human zona pellucida from fertilized and unfertilized eggs. J Reprod Fertil 82:151–161
Stewart-Savage J, Bavister BD (1988) A cell surface block to polyspermy occurs in golden hamster eggs. Dev Biol 128:150–157
Tian J, Gong H, Thomsen GH et al (1997) Gamete interactions in Xenopus laevis: identification of sperm binding glycoproteins in the egg vitelline envelope. J Cell Biol 136:1099–1108
Yauger B, Boggs N, Dean J (2011) Human ZP4 is not sufficient for taxon-specific sperm binding to the zona pellucida in transgenic mice. Reproduction 141:313–319
Acknowledgments
The critical reading of the manuscript by Dr. Matteo Avella is appreciated. This research was support by the Intramural Research Program of the National Institutes of Health, NIDDK.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Dean, J. (2014). A ZP2 Cleavage Model of Gamete Recognition and the Postfertilization Block to Polyspermy. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_33
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_33
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)