Abstract
A typical ejaculate contains more than 100 million spermatozoa; however, only 1 spermatozoon participates in fertilization. There might be an ingenious molecular mechanism to ensure that a spermatozoon fertilizes an egg. Recent gene disruption experiments in mice have revealed that there are two key factors in the sperm–egg fusion process. CD9 on eggs and IZUMO1 on spermatozoa have emerged as indispensable factors. However, the molecular mechanism of sperm–egg fusion, that is, how and when sperm–egg fusion occurs, remains virtually unknown. We have recently reported the dynamics of redistribution of IZUMO1 during the acrosome reaction using red fluorescent protein-tagged IZUMO1 for live imaging of the moment of sperm–egg fusion. Consequentially, the result suggests that IZUMO1 diffused from the acrosomal membrane to the sperm surface in the equatorial segment, which is considered to initiate fusion with the oocyte; fusion takes place after the acrosome reaction. This was the first evidence for live imaging to monitor the fusion-related protein at sperm–egg fusion.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Mammalian fertilization is the phenomenon in which a spermatozoon and egg find each other, interact, and fuse. There are many obstacles, including migration into the oviduct and penetration into the cumulus layer and zona pellucida, for the spermatozoa before reaching the egg. This phenomenon is essential for sexual reproduction. Although many experiments have been performed and papers published about the biological importance of fertilization so far, the molecular mechanism of fertilization remains substantially unknown. However, gene-knockout experiments made us aware of the existence of essential genes.
In the sperm–egg fusion process, only two key factors, a member of the immunoglobulin superfamily, IZUMO1, in the spermatozoon and a member of the tetraspanin family, CD9, in the oocyte, have been identified to be essential (Inoue et al. 2005; Miyado et al. 2000; Le Naour et al. 2000; Kaji et al. 2000). Loss of these factors results in sterility, but currently there is no evidence that they act as direct fusogenic proteins. However, the fact that the requirement for these factors can be restored by bypassing the fusion step via intracytoplasmic sperm injection (ICSI) suggests that they make specific contributions to egg–sperm fusion (Inoue et al. 2005).
IZUMO1 was initially identified by a sperm–egg fusion inhibitory monoclonal antibody. IZUMO1-deficient spermatozoa appear morphologically normal, and bind and penetrate the zona pellucida surrounding the egg, but are not capable of fusing with eggs (Inoue et al. 2005). IZUMO1 is initially hidden in the acrosomal organelle under the plasma membrane. After the acrosome reaction, it relocates to the surface of the sperm head, suggesting that redistribution of IZUMO1 is essential for fusion (Inoue et al. 2005; Satouh et al. 2012).
CD9 was shown to be required for fusion on the egg plasma membrane (Miyado et al. 2000; Le Naour et al. 2000; Kaji et al. 2000). It was proposed that exosome-like CD9-containing vesicles are secreted from unfertilized eggs, thereby conferring fusion competence to the spermatozoon (Miyado et al. 2008). In addition to the sterility phenotype, the length, thickness, and density of the microvilli of the eggs of CD9-deficient mice are altered, suggesting that CD9 participates in microvilli formation and that microvilli are important for sperm–egg fusion (Runge et al. 2007). CD9 has also been suggested to be involved in adhesion of the membrane to trigger sperm–egg fusion (Jégou et al. 2011).
There is no evidence indicating that IZUMO1 and CD9 directly interact during sperm–egg fusion. Even though IZUMO1 and CD9 are essential for sperm–egg fusion, it is still unclear how they participate in the process and if other proteins are involved.
2 IZUMO1 Is an Essential Protein for Sperm–Egg Fusion
We produced the anti-mouse spermatozoon monoclonal antibody, OBF13, that inhibits the fusion process (Okabe et al. 1987; Okabe et al. 1988). The antigen recognized by OBF13 was not identified for many years. However, it was recently identified by two-dimensional gel electrophoresis and subsequent immunoblotting and liquid chromatography-tandem mass spectroscopy analysis. We named the antigen “Izumo” after a Japanese shrine dedicated to marriage. The gene encodes a novel immunoglobulin superfamily type I membrane protein with an extracellular Ig domain. Recently, according to Ellerman et al. (Ellerman et al. 2009), IZUMO proteins consist of four family proteins (IZUMO1 to IZUMO4). The N-terminal domain between signal peptides and the Ig domain showed a significant homology to each other and was termed the Izumo domain.
After producing Izumo1 −/− mutant mice, we found that they were healthy and showed no overt developmental abnormalities. As expected, Izumo1 −/− male mice became sterile although they exhibited normal mating behavior and ejaculated to form normal vaginal plugs. Moreover, the sperm penetrated the zona pellucida without any problem but failed to fuse with the eggs, causing an accumulation of spermatozoa in the perivitelline space of the eggs (Inoue et al. 2005) (Fig. 32.1).
We also examined the acrosomal status of Izumo1 −/− spermatozoa. To verify the acrosome reaction, we stained the spermatozoa with an MN9 monoclonal antibody, which stains only to the equatorial segment of acrosome-reacted spermatozoa (Toshimori et al. 1998) (Fig. 32.1). As shown in Fig. 32.1, Izumo1 −/− spermatozoa were clearly stained for MN9. This finding indicated that Izumo1 −/− spermatozoa had undergone the acrosome reaction but failed to fuse with eggs.
We further examined whether the defect of Izumo1 −/− spermatozoa is limited to their fusing ability with eggs or whether it extends to later developmental stages. To address this question, we injected Izumo1 −/− spermatozoa directly into the cytoplasm of wild-type eggs and observed the ability of later development. Eggs injected with Izumo1 −/− spermatozoa were successfully activated and implanted normally. The embryos developed to term in a normal ratio (Inoue et al. 2005).
3 The Role of N-Glycosylation in IZUMO1
IZUMO1 possesses an N-glycosylation site, which is well conserved among species, in the middle of an Ig loop. This site must be glycosylated because if we incubated mouse IZUMO1 from spermatozoa with N-glycosidase, the molecular weight of IZUMO1 decreased from its original size of 56 to 50 kDa. Because glycan composition is known to be involved in many molecular interaction mechanisms (Ohtsubo and Marth 2006), we attempted to examine the role of N-glycan on IZUMO1. To answer this question, we produced mouse lines expressing mutated IZUMO1. In particular, residue 204, asparagine, in the putative N-glycosylation site, was substituted with glutamine by site-directed mutagenesis under the testis-specific Calmegin promoter with the rabbit beta-globin polyadenylation signal. After we established N204Q-IZUMO1 male mice, we crossed this transgenic mouse line with Izumo1 −/− mice and produced a mouse line that has spermatozoa with no N-glycosylation site in IZUMO1. Although the litter sizes were smaller compared to wild-type IZUMO1 mice, the infertile phenotype was rescued in N204Q-IZUMO1 mice. The efficiency was low, but spermatozoa from N204Q-IZUMO1 mice could fuse with eggs. We extracted proteins from testis and spermatozoa from N204Q-IZUMO1 mice and analyzed IZUMO1 by Western blot analysis. N204Q-IZUMO1 from testis appeared in the 50-kDa area because of the lack of N-linked glycan. However, severe fragmentation was observed for N204Q-IZUMO1 from spermatozoa, which was not observed in wild-type IZUMO1. The majority of fragmented bands was observed in the ~30- and ~35-kDa areas. Although N204Q-IZUMO1 could rescue the infertile phenotype, the amount of intact N204Q-IZUMO1 present in spermatozoa was significantly smaller compared to that of wild-type IZUMO1, in spite of an abundance of N204Q-IZUMO1 in testis (Inoue et al. 2008). This finding indicates that glycosylation is not essential for the function of IZUMO1 but that it has a role in protecting IZUMO1 from fragmentation in the cauda epididymis.
4 IZUMO1-Interacting Protein
Because IZUMO1 has no “fusogenic” peptide or “SNARE”-like structure in it, we considered the possibility that IZUMO1 might be one of the components forming a fusogenic machinery on spermatozoa. To search for IZUMO1-interacting proteins, we made a transgenic mouse line producing IZUMO1-His on spermatozoa and introduced it to an Izumo1 −/− background, which allowed us to immunoprecipitate IZUMO1 using an anti-His antibody. The IZUMO1-interacting protein was purified from acrosome-intact sperm lysates by using anti-His microbeads. We could detect a specific 80-kDa band in the purified fraction by silver staining. After liquid chromatography-tandem mass spectroscopy analysis, the protein was identified as ACE3 (angiotensin I-converting enzyme 3) (Rella et al. 2007).
We generated Ace3-deficient mice by homologous recombination. Differing from our expectation, Ace3 −/− mice showed signs of infertility in both males and females. We analyzed the fertilizing ability of Ace3 −/− spermatozoa in an in vitro fertilization system. Again, Ace3 −/− spermatozoa showed normal fertilizing ability in our in vitro fertilization system using both cumulus-intact and cumulus-free eggs. These results suggest that ACE3 binds to IZUMO1, but this characteristic nature is not required for spermatozoa to fertilize eggs (Inoue et al. 2010).
5 Dynamic Translocation of IZUMO1
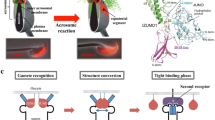
IZUMO1 is not exposed on the freshly prepared sperm surface, and it appears on the plasma membrane after acrosome reaction. It is not known how IZUMO1 appears on the plasma membrane or how it behaves at the moment of sperm-egg fusion. To visualize the behavior of IZUMO1 in living spermatozoa, we generated a transgenic mouse line expressing mCherry-tagged IZUMO1 (Red-IZUMO1) using a testis-specific promoter and optimized a confocal microscope system with ultralow invasiveness. By using Red-IZUMO1 transgenic mice in combination with Izumo1 −/− mice and Green-acrosome transgenic mice that overexpress GFP specifically in the acrosome (Nakanishi et al. 1999), we examined the precise localization of IZUMO1 before and after acrosome reaction as well as subsequent fertilization processes. The physiological function of Red-IZUMO1 was proven in a gene rescue experiment. Red-IZUMO1 was localized on both inner and outer acrosomal membranes in freshly prepared spermatozoa. At the time of acrosome reaction, Red-IZUMO1 demonstrated rapid diffusion onto the whole sperm head and then tended to localize in the medial region of the sperm head, called the equatorial segment (Fig. 32.2a,b). The moment of sperm–egg fusion was also imaged live through Red-IZUMO1, demonstrating that the diffusion of Red-IZUMO1 onto the egg membrane starts at the equatorial segment (Fig. 32.2c). Further experimentation using CD9-GFP eggs indicated that IZUMO1 on the inner acrosomal membrane is engulfed into the egg cytoplasm. In this study, we visualized the dynamic movement of the fusion-related protein IZUMO1 during fertilization processes. We found that IZUMO1 migrated out through fusion pores formed between the plasma membrane and outer acrosomal membrane and that it diffused away from the equatorial segment to the egg plasma membrane at the beginning of sperm–egg fusion (Satouh et al. 2012).
Visualization of sperm–egg fusion. (a) The sperm head consists mainly of three subcellular regions. Scanning electron micrographic view of the sperm head indicates each region: acrosomal cap (AC), equatorial segment (EQ), and postacrosomal region (PA). (b) Fluorescent images of spermatozoa from Red-IZUMO1 and Green-acrosome double-transgenic mouse. IZUMO1 was initially localized in both the outer membrane and inner acrosomal membrane in the acrosomal cap but not in the equatorial segment. Thus, acrosome-intact spermatozoa have Red-IZUMO1 in the acrosomal cap area with GFP, whereas Red-IZUMO1 spread to the entire head, including the equatorial segment, after acrosome reaction (GFP negative). (c) A representative time-lapse view of sperm–egg fusion. Initiation of fusion (time 0) was detected by diffusion of Red-IZUMO1 from the equatorial segment and the concomitant transfer of Hoechst 33342 dye to spermatozoa in the same area (arrowheads). Diffusion of the membrane in the postacrosomal area identified by ADAM1B (green) started at 60 s after the initiation of sperm–egg fusion, and this was accompanied by expansion of the Hoechst 33342 staining area toward the posterior head. After the fusion process, IZUMO1 is only present in the inner acrosomal membrane (asterisk)
6 Perspective
A partial molecular mechanism of fertilization has been clarified by gene-manipulated animal experiments. However, to elucidate detailed mechanism leading to a new schema, it is necessary to analyze the properties of each factor as well. Concerning the membrane fusion of spermatozoon and oocyte that is the central event of fertilization, targeted deletion studies have revealed only two proteins, CD9 on oocyte and IZUMO1 on spermatozoon, to be necessary in sperm–egg fusion. If all the essential components and molecular behaviors of the fusion machinery are sufficiently identified, elucidation of the sperm–egg fusion mechanism will be greatly promoted. Also, this result might prompt us to thoroughly understand the fundamental principle of wider areas of the cell–cell fusion process such as the formation of myotubes, placenta, multinucleated osteoclasts, and macrophages. Clarification of the molecular mechanism of fertilization will benefit clinical treatment of sterility and will support the potential development of novel contraceptive strategies in the future.
References
Ellerman DA, Pei J, Gupta S, Snell WJ, Myles D, Primakoff P (2009) Izumo is part of a multiprotein family whose members form large complexes on mammalian sperm. Mol Reprod Dev 76:1188–1199
Inoue N, Ikawa M, Isotani A, Okabe M (2005) The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature (Lond) 434:234–238
Inoue N, Ikawa M, Okabe M (2008) Putative sperm fusion protein IZUMO and the role of N-glycosylation. Biochem Biophys Res Commun 377:910–914
Inoue N, Kasahara T, Ikawa M, Okabe M (2010) Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. PLoS One 5:e10301
Jégou A, Ziyyat A, Barraud-Lange V, Perez E, Wolf JP, Pincet F, Gourier C (2011) CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc Natl Acad Sci USA 108:10946–10951
Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A (2000) The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 24:279–282
Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C (2000) Severely reduced female fertility in CD9-deficient mice. Science 287:319–321
Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E (2000) Requirement of CD9 on the egg plasma membrane for fertilization. Science 287:321–324
Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A (2008) The fusing ability of sperm is bestowed by CD9-containg vesicles released from eggs in mice. Proc Natl Acad Sci USA 105:1292–1296
Nakanishi T, Ikawa M, Yamada S, Parvinen M, Baba T, Nshimune Y, Okabe M (1999) Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett 449:277–283
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126:855–867
Okabe M, Adachi T, Takada K, Oda H, Yagasaki M, Kohama Y, Mimura T (1987) Capacitation-related changes in antigen distribution on mouse sperm heads and its relation to fertilization rate in vitro. J Reprod Immunol 11:91–100
Okabe M, Yagasaki M, Oda H, Matzno S, Kohama Y, Mimura T (1988) Effect of a monoclonal anti-mouse sperm antibody (OBF13) on the interaction of mouse sperm with zona-free mouse and hamster eggs. J Reprod Immunol 13:211–219
Rella M, Elliot JL, Revett TJ, Lanfear J, Phelan A, Jackson RM, Turner AJ, Hooper NM (2007) Identification and characterisation of the angiotensin converting enzyme-3 (ACE3) gene: a novel mammalian homologue of ACE. BMC Genomics 8:194
Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG (2007) Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol 304:317–325
Satouh Y, Inoue N, Ikawa M, Okabe M (2012) Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci 125:4985–4990
Toshimori K, Saxena DK, Tanii I, Yoshinaga K (1998) An MN9 antigenic molecule, equatorin, is required for successful sperm-oocyte fusion in mice. Biol Reprod 59:22–29
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (21112006 and 21687018).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Inoue, N. (2014). The Mechanism of Sperm–Egg Fusion in Mouse and the Involvement of IZUMO1. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_32
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_32
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)