Abstract
Malaria, caused by Plasmodium spp., is transmitted by anopheline mosquitoes. When male and female gametes are introduced into the mosquito midgut, they reproduce sexually and proliferate, at which time the mosquito becomes infectious to vertebrates. It has been proposed that fertilization is a critical target in the parasite life cycle for the reduction of malarial prevalence. Although understanding parasite fertilization is crucial for the control of malaria, the precise molecular mechanisms involved have long remained unknown. Generative cell-specific 1 (GCS1) has been reported to be a critical fertilization factor in angiosperms. It was subsequently shown that the function of GCS1 is conserved in both the rodent malaria parasite Plasmodium berghei and the green alga Chlamydomonas reinhardtii. Moreover, a GCS1-like gene has been detected in the genomes of various organisms, suggesting that it plays a conserved role in gamete interaction. As GCS1 is thought to act as a membrane-anchoring protein in male gametes, a female counterpart is assumed to exist. To reveal the mechanisms involved in parasite fertilization, it is important to clarify the function of GCS1 and to identify GCS1 partners and other fertilization factors. In this review, I first describe the life cycle of malaria parasites, focusing on gametogenesis and fertilization and the underlying mechanisms. I then discuss the functions of GCS1 at the time of gamete interaction. Finally, I consider whether parasite fertilization factors, including GCS1, might be utilized in the development of antimalarial vaccines.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Sexual Differentiation and Gametogenesis of Malaria Parasites
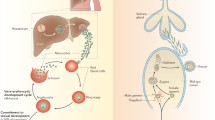
In vertebrates, mature malaria parasites infect erythrocytes by egressing from older cells and invading newer ones. In asexually proliferating parasites, a subset of the parasites escapes from the asexual cycle and differentiate to produce male and female gametocytes (gametocytogenesis). When these gametocytes are introduced into the midgut of mosquitoes through feeding on malaria-infected vertebrates, they differentiate into gametes (gametogenesis). During gametogenesis, gametocytes shed the erythrocytic membrane within a few minutes of mosquito feeding. Subsequently, male gametes undergo three rounds of DNA replication within 2 to 10 min. Between 10 and 12 min, eight motile flagella (the motile form of male gametes) are released (Sinden et al. 1996) in a phenomenon termed exflagellation (Ross and Smyth 1997). For Plasmodium, an in vitro fertilization (IVF) assay has been established involving three factors required to induce gametogenesis: (1) a drop in temperature from 37 °C to 21 °C; (2) the presence of xanthurenic acid (XA), a previously determined mosquito-derived factor (Billker et al. 1998); and (3) alkaline conditions (pH 8.0). Through IVF, it has been demonstrated that XA activates membrane-bound guanylate cyclase (GC) (Muhia et al. 2001) and cGMP-dependent protein kinase (PKG) (McRobert et al. 2008), which are essential for male exflagellation. Moreover, N-methyl-hydroxylamine, an inhibitor of GC, inhibits exflagellation (Kawamoto et al. 1990). These findings demonstrate that cGMP is involved in XA-mediated signaling. In addition to cGMP signaling, it has been reported that intracellular Ca2+ concentrations in gametocytes are increased within 10 s after XA stimulation. Parasites lacking the calcium-dependent protein kinase 4 gene are defective in genomic replication and mitosis, resulting in an absence of exflagellation (Billker et al. 2004). This finding suggests that XA may also activate calcium signaling. Furthermore, it has been reported that P. berghei homologues of atypical MAP kinase 2 (Map-2) (Rangarajan et al. 2005; Tewari et al. 2005) and cdc20 (Guttery et al. 2012) are required for male gametogenesis. Although the aforementioned molecules have been demonstrated to be involved in XA-mediated signal transduction, the most important question remains unanswered: How does XA induce gametogenesis? Given that membrane-bound GC in gametocytes is activated by XA in vitro (Muhia et al. 2001), a membrane-coupled XA receptor may exist to activate PKG and calcium signaling, thereby triggering gametogenesis.
2 Recognition, Attachment, and Membrane Fusion of Plasmodium Gametes
In general, the process of fertilization can be divided into three steps: recognition, attachment, and membrane fusion of gametes. In malaria parasites, it has been shown that male flagella swim freely, exhibiting no directed movement toward female gametes in vitro (Sinden 1983). Therefore, although many plants and animals utilize chemoattractants for gamete recognition when they are spatially isolated, malaria parasites may not possess such a system. Nevertheless, Plasmodium gametes must possess a molecular system for species-specific recognition. A recent study demonstrated that the gamete membrane-surface proteins P48/45, P47, and P230, which belong to the 6-cys family, are critical in gamete recognition. Mutant parasites lacking either P48/45 or P47 and P230 show male or female subfertility, respectively (van Dijk et al. 2010). Further phenotypic observation revealed that these mutants are unable to either recognize or attach to each other, demonstrating the vital role of this family of proteins in gamete recognition/attachment. Importantly, the fertility of these mutants is not completely impaired, suggesting that alternative pathways may compensate for the function of these proteins. Thus, definitive fertilization factors for malaria parasites have not been identified until recently.
In Plasmodium, the male gametes swim in a fashion similar to mammalian sperm and attach vigorously to female gametes. Interestingly, after entering female cells, the male gametes continue flagellar movement inside the cell for 1 min after fusion (Sinden and Croll 1975). A similar phenomenon has been observed in the fruit fly Drosophila melanogaster, whose spermatic membrane is detected in eggs until immediately after fertilization. Sneaky (Snky), a sperm membrane-specific protein, is responsible for sperm membrane degradation and is essential for successful fertilization (Wilson et al. 2006). Putative Snky homologues are present in vertebrate species, implying their functional conservation (Wilson et al. 2006). However, Plasmodium species do not possess an obvious homologue of this protein, suggesting that the molecular mechanism of Plasmodium fertilization is, at least in part, distinct from that of animals.
3 GCS1: An Ancient Fertilization Factor Conserved in Animals and Plants
Mori et al. reported a male gamete-specific protein, designated generative cell-specific 1 (GCS1), as a novel fertilization factor in angiosperms (Mori et al. 2006). GCS1 is a putative single-pass transmembrane protein with a putative N-terminal signal sequence but no functional domain (Mori et al. 2006; von Besser et al. 2006). GCS1 knockout (KO) Arabidopsis thaliana plants show a severe form of male sterility in which male gametes can access the egg cell normally but cannot fuse with female gametes, suggesting that only the gamete interaction process is impaired in GCS1 KO plants (Mori et al. 2006; von Besser et al. 2006). Interestingly, functional GCS1 homologues are present in green algae and rodent malaria parasites (Mori et al. 2006; von Besser et al. 2006; Liu et al. 2008; Hirai et al. 2008). Similar to angiosperm GCS1s, the expression of P. berghei GCS1 (PbGCS1) shows male gamete specificity, and loss of PbGCS1 results in male sterility, whereas female gametes remain fertile (Hirai et al. 2008; Liu et al. 2008). This situation is in sharp contrast to that in mutant parasites lacking any of the 6-cys family proteins, whose fertility is only partially impaired, suggesting that no complementary molecule for GCS1 exists in the parasite. In green algae, Liu et al. demonstrated the exact timing of GCS1 function. Algal GCS1 acts during the few seconds between the pre-fusion adhesion and fusion steps, providing evidence that GCS1 may play a role in gamete membrane fusion following gamete recognition (Liu et al. 2008). It is generally accepted that the molecules that function in species recognition evolve rapidly, leading to reproductive isolation and speciation among eukaryotes (Swanson and Vacquier 2002). In this context, GCS1 has been shown to be conserved in various organisms, and the functional timing observed during gamete recognition suggests that GCS1 may have a role in membrane fusion following species recognition. A previous study by our group demonstrated that male gametes lacking PbGCS1 can attach to their female counterparts, suggesting that PbGCS1 is unnecessary for gamete recognition (Hirai et al. 2008). To further confirm that GCS1 is not involved in gamete recognition, we generated transgenic P. berghei in which the endogenous PbGCS1 gene was replaced with that of P. yoelii (PyGCS1), another rodent malaria parasite. Because P. berghei cannot fertilize P. yoelii, the PyGCS1-expressing P. berghei male gametes are expected to fail in the fertilization of wild-type (WT) P. berghei female gametes if the GCS1 protein plays any role in species-specific gamete recognition. Our results clearly demonstrated that the transgenic P. berghei male gametes could fertilize WT P. berghei female gametes, indicating that Plasmodium GCS1 is not involved in gamete recognition. Moreover, heterogeneous expression of PyGCS1 in P. berghei did not result in any adverse effects on parasite development, even after the zygote stage, suggesting functional complementation of PyGCS1 in P. berghei (Hirai et al., unpublished data).
Given that GCS1 contains no predictable functional motif except for a signal sequence and transmembrane domain, there are no available clues to allow speculation regarding its function. As an initial step toward understanding the function of PbGCS1, we generated a series of transgenic parasites in which endogenous PbGCS1 was partially deleted to characterize the critical functional domains of the PbGCS1 protein. Our results revealed that the putative cytosolic region in the C-terminus of the protein is not necessary for successful fertilization but that all other regions (e.g., the single-peptide transmembrane domain and recently defined motifs such as HAP2/GCS1) are essential. Taken together, the results of this study revealed that functional domains of PbGCS1 involved in gamete fusion reside in putative extracellular locations and that PbGCS1 must be localized to the cell surface to function properly (Mori et al. 2010). Interestingly, a similar analysis was performed for Arabidopsis GCS1, generating results similar to those found for PbGCS1 (Mori et al. 2010) and suggesting a conserved topology of GCS1 among GCS1-possessing organisms. Additionally, the extracellular localization of GCS1 allows us to speculate that GCS1 may function on the surface of male gametes by interacting with a partner molecule on female gametes. It is clear that the identification of GCS1 partners and other fertilization factors will accelerate our understanding of fertilization systems not only for malaria parasites but also for all GCS1-possessing organisms.
4 Application of Fertilization Factors to Anti-Malarial Vaccine Development
Malaria is a serious disease that was responsible for 655,000 deaths and 216 million reported cases in 2010 alone (Murray et al. 2012). The emergence of parasites that are resistant to drugs and vaccines necessitates the development of new strategies for malaria control. However, the development of antimalarial vaccines and drugs is difficult because both the antibodies elicited by vaccination and drugs provide selective pressure and induce recombination/mutation in the parasite genome, resulting in antigenic polymorphisms and the emergence of parasites that are tolerant to these treatments. In addition to antimalarial strategies aimed at the human blood-stage parasite, a transmission-blocking vaccine (TBV) that attacks the insect-stage parasite in mosquitoes has been proposed. Mosquitoes do not exhibit adaptive immunity and rely solely on innate immunity (Faye 1990), suggesting that insect-stage parasites do not face antibody-based immune pressure. It has therefore been speculated that an antibody-based immune attack could be effective against mosquito-stage parasites because they may not evolve any strategy for combating antibodies during the mosquito stage. Based on this assumption, TBV candidates have been selected from molecules expressed in gametocytes, gametes, zygotes, and ookinetes. Several cell-surface molecules (Pfs230, P48/45, P25/28) have been tested as TBV targets thus far, and the antibodies raised against them have succeeded in reducing the rate of parasite transmission (Barr et al. 1991; Healer et al. 1999; Williamson 2003). However, none of these studies has demonstrated complete blockage of transmission, most likely because the TBV candidate molecules are quite abundant in parasites and because the small quantity of antibodies administered may not be sufficient to completely block the activity of the candidate molecules. Even if the antibodies were able to fully block this activity, it is possible that the parasites would still be fertile, because parasites lacking any of these genes can still reproduce sexually in mosquitoes (van Dijk et al. 2010). Therefore, other molecules that display a definitive and crucial function in sexual reproduction represent ideal TBV candidates. We believe GCS1 best fits this criterion because PbGCS1 protein expression levels are quite low in the parasite, and a lack of PbGCS1 protein completely abrogates parasite fertility (Khan et al. 2005; Hirai et al. 2008). Recently, Blagborough et al. and our group performed experiments demonstrating that an anti-PbGCS1 antibody significantly, but not completely, blocked parasite reproduction based on IVF assays (Blagborough et al. 2013; Hirai et al. unpublished data). This failure to completely inhibit reproduction may arise from PbGCS1 possessing many cysteine residues and displaying a complex conformation that is not recapitulated by the recombinant protein, resulting in less effective antibody production. Such a requirement for correct folding of recombinant TBV proteins for effective antibody production has been observed in cases in which the TBV candidate is produced in yeast and algal systems (Kaslow et al. 1994; Gregory et al. 2013). In addition to this problem, the immunization protocol needs to be improved to generate higher antibody titers (Kubler-Kielb et al. 2007; Outchkourov et al. 2008). It is reasonable to assume that the use of multiple TBV targets would be effective because the antibodies raised would simultaneously neutralize or block multiple parasite molecules, leading to complete blockage of parasite fertilization. Thus, we have employed a transcriptomic approach to identify new fertilization factors for malaria parasites, and TBV candidates will be screened from this source.
5 Concluding Remarks
Since the draft genome sequences of the human malaria parasite, P. falciparum, and its vector, Anopheles gambiae, have been published and reverse genetics has been applied to the parasite, our knowledge of malaria biology has expanded. However, this infectious disease is still life threatening. As I mentioned earlier, the main reason for failure in eradication of this disease is the appearance of parasites tolerant to drug and vaccine treatments, and we need to find new ways to eradicate this disease. In this context, TBV might be a new weapon to combat the parasites. Because parasite fertilization is the first stage in the mosquito life cycle, it is assumed that the parasite fertilization factor would be the most effective TBV candidate. We understand that there are many hurdles to overcome before a long-lasting and highly effective TBV is obtained. Nevertheless, we believe that our attempt will result in not only a discovery of new TBV but also in clearer understanding of the molecular mechanisms underlying malarial parasite sexual reproduction.
References
Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC (1991) Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174:1203–1208
Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR (1998) Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature (Lond) 392:289–292
Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V (2004) Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117:503–514
Blagborough AM, Churcher TS, Upton LM, Ghani AC, Gething PW, Sinden RE (2013) Transmission-blocking interventions eliminate malaria from laboratory populations. Nat Commun 4:1812
Faye I (1990) Acquired immunity in insects: the recognition of nonself and the subsequent onset of immune protein genes. Res Immunol 141:927–932
Gregory JA, Topol AB, Doerner DZ, Mayfield S (2013) Algae-produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl Environ Microbiol 79(13):3917–3925
Guttery DS, Ferguson DJ, Poulin B, Xu Z, Straschil U, Klop O, Solyakov L, Sandrini SM, Brady D, Nieduszynski CA et al (2012) A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development. PLoS Pathog 8:e1002554
Healer J, McGuinness D, Carter R, Riley E (1999) Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology 119(pt 5):425–433
Hirai M, Arai M, Mori T, Miyagishima SY, Kawai S, Kita K, Kuroiwa T, Terenius O, Matsuoka H (2008) Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr Biol 18:607–613
Kaslow DC, Bathurst IC, Lensen T, Ponnudurai T, Barr PJ, Keister DB (1994) Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun 62:5576–5580
Kawamoto F, Alejo-Blanco R, Fleck SL, Kawamoto Y, Sinden RE (1990) Possible roles of Ca2+ and cGMP as mediators of the exflagellation of Plasmodium berghei and Plasmodium falciparum. Mol Biochem Parasitol 42:101–108
Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M, Waters AP (2005) Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121:675–687
Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, Shiloach J, Robbins JB, Schneerson R (2007) Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci USA 104:293–298
Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ et al (2008) The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22:1051–1068
McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA (2008) Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol 6:e139
Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T (2006) Generative cell specific 1 is essential for angiosperm fertilization. Nat Cell Biol 8:64–71
Mori T, Hirai M, Kuroiwa T, Miyagishima SY (2010) The functional domain of GCS1-based gamete fusion resides in the amino terminus in plant and parasite species. PloS One 5:e15957
Muhia DK, Swales CA, Deng W, Kelly JM, Baker DA (2001) The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum. Mol Microbiol 42:553–560
Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431
Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D, van Gemert GJ, van de Vegte-Bolmer M, Sauerwein RW, Stunnenberg HG (2008) Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci USA 105:4301–4305
Rangarajan R, Bei AK, Jethwaney D, Maldonado P, Dorin D, Sultan AA, Doerig C (2005) A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei. EMBO Rep 6:464–469
Ross R, Smyth J (1997) On some peculiar pigmented cells found in two mosquitoes fed on malarial blood. 1897. Indian J Malariol 34:47–55
Sinden RE (1983) Sexual development of malarial parasites. Adv Parasitol 22:153–216
Sinden RE, Croll NA (1975) Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology 70:53–65
Sinden RE, Butcher GA, Billker O, Fleck SL (1996) Regulation of infectivity of Plasmodium to the mosquito vector. Adv Parasitol 38:53–117
Swanson WJ, Vacquier VD (2002) The rapid evolution of reproductive proteins. Nat Rev Genet 3:137–144
Tewari R, Dorin D, Moon R, Doerig C, Billker O (2005) An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol 58:1253–1263
van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, van Gemert GJ, Kroeze H, Stunnenberg HG, Eling WM et al (2010) Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog 6:e1000853
von Besser K, Frank AC, Johnson MA, Preuss D (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development (Camb) 133:4761–4769
Williamson KC (2003) Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol 25:351–359
Wilson KL, Fitch KR, Bafus BT, Wakimoto BT (2006) Sperm plasma membrane breakdown during Drosophila fertilization requires sneaky, an acrosomal membrane protein. Development (Camb) 133:4871–4879
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (24112705) a grant for research into emerging and re-emerging infectious diseases from the Ministry of Health, Labor and Welfare of Japan (H23-Shinkosaiko-ippan-014; http://www.mhlw.go.jp/english/).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Hirai, M. (2014). Fertilization Mechanisms of the Rodent Malarial Parasite Plasmodium berghei . In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_27
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_27
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)