Abstract
Coffee leaf rust (CLR), caused by the obligate biotrophic fungus Hemileia vastatrix, is considered one of the most devastating diseases of Arabica coffee. The use of leaf rust resistant or tolerant coffee varieties is a critical component for effective management of this disease at the farm level. Conventional breeding of Arabica coffee for leaf rust resistance requires many years of breeding and field-testing. Induced mutagenesis is an effective tool to increase genetic variability and generate new alleles with potential benefit for addressing abiotic and biotic stresses such as leaf rust in Arabica coffee. Efficient screening methods are required to evaluate coffee germplasm or mutant populations for resistance to H. vastatrix. Here, we present a screening method that uses inoculation of leaf discs in a controlled environment. The method was evaluated using M1V1 and M2 plants derived from chemically mutagenized Arabica coffee cell suspensions. In this method, the first rust symptoms appear on the leaf discs approximately 29 days after inoculation while the disease severity and incidence can be scored about 47 days after inoculation. Our results show that the methodology is simple, efficient and suitable to rapidly screen large mutant populations in a small area.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Coffee (Coffea arabica L.) is one of the most important beverages in the world and the second most important commercial product exported by developing countries (Alemayehu 2017). Coffee leaf rust (CLR), caused by the biotrophic fungus Hemileia vastatrix Berk. and Broome, is one of the main limiting factors of Arabica coffee production worldwide (Waller et al. 2007). The disease can reduce global coffee production by 20 to 25%, with losses of over $ 1 billion annually (McCook 2006; Talhinhas et al. 2017).
The application of fungicides has been the most widely used method to control CLR, even when the development of varieties with genetic resistance is the best alternative (Zambolim 2016). The quest for natural resistance to CLR by traditional breeding has been the focus of research for decades (Melese Ashebre 2016; Mishra and Slater 2012). However, conventional genetic control of CLR has been hampered by the prodigious pathological diversity and rapid genetic evolution of the fungus overcoming the plant resistance genes deployed so far (Cabral et al. 2016; Lima et al. 2020). The induction of genetic variability in Arabica coffee through mutagenesis provides an important complementary tool for crop improvement programs, since a range of variants can be generated (Dhumal and Bolbhat 2012; Vargas-Segura et al. 2019).
Chemical mutagens such as sodium azide (NaN3) and ethyl methanesulfonate (EMS), have been used in crop breeding for developing mutants (Bolívar-González et al. 2018; Laskar et al. 2018). These chemical mutagens induce a broad variation of morphological and yield-related traits. Other authors reported cases of crops treated with chemical mutagens and improved for fungal resistance or tolerance, for example, powdery mildew-resistant barley (Khan et al. 2010) and wheat resistant to leaf rust Puccinia sp. (Mago et al. 2017).
Genetic studies related to H. vastatrix and coffee genotypes pursuing resistance, require periodic inoculation of different uredospores of the fungus into the host. A safe and efficient way to evaluate the resistance to different H. vastatrix races is carried out by infection in situ, using detached leaves or leaf discs under controlled conditions of humidity, light, and temperature that stimulate the development of the pathogen (Cabral et al. 2016; Eskes 1982).
This chapter presents a Coffee leaf rust resistance screening method based on inoculation of leaf discs under controlled conditions. The method was evaluated using M1V1 mutant plants obtained from M0 embryogenic callus treated with NaN3 and EMS and the resulting M2 population. The method proved suitable to rapidly screen large coffee populations for CLR resistance.
2 Materials
2.1 Plant Material
-
1.
Coffea arabica. var. Catuaí plants M1V1 (see Note 1).
-
2.
Coffea arabica var. Catuaí seeds M2 (see Note 2).
-
3.
C. arabica var. Obatá (or any other CLR resistant variety).
-
4.
C. arabica var. Caturra (or any other CLR susceptible variety).
-
5.
C. canephora (or any other CLR resistant species).
2.2 Other Biological Materials
-
1.
Coffee leaves with rust spores.
-
2.
Healthy coffee leaves (in greenhouse).
2.3 Consumables and Minor Equipment
-
1.
Black polyethylene bags (6 × 8 in).
-
2.
Calibrated scoops for fertilizer application.
-
3.
Commercial potting soil.
-
4.
Cylindrical punch (10 mm-diameter) (e.g., Korff model 06940-5/16).
-
5.
Falcon tube (50 ml).
-
6.
Latex gloves.
-
7.
Microcentrifuge tubes (1.5 ml).
-
8.
Mix of screened compost.
-
9.
Napkins.
-
10.
Permanent markers.
-
11.
Plant labels (with progenies number, plant number).
-
12.
Plastic boxes (12 × 10 × 3.5 cm).
-
13.
Plastic dropper.
-
14.
Plastic grid.
-
15.
Plastic pots (1–2 L capacity).
-
16.
Rice husk.
-
17.
Scalpels blades.
-
18.
Scalpels.
-
19.
Shovels.
-
20.
Slow-release fertilizer.
-
21.
Soil.
-
22.
Wheelbarrow.
2.4 Reagents and Agrochemicals
-
1.
Bayfolan forte (10 ml/L) (Bayer S.A, Amatitlán, Guatemala).
-
2.
Distilled water.
-
3.
Fungicide Vitavax 40 WP (Chemtura Corporation, Middlebury, USA).
-
4.
Osmocote Pro, 19:9:10 + 2MgO + TE.
-
5.
Tween 20 (Research Products International, Illinois, USA).
2.5 Equipment
-
1.
LED light lamps (Heliospectra, model LX 602).
-
2.
Microscope.
-
3.
Neubauer chamber or Haemocytometer slide.
-
4.
Stereo microscope.
-
5.
Incubator with light, humidity, and temperature control (e.g., BIOBASE brand, model BJPX-L400.Shandong, China).
-
6.
Electronic Digital Vernier Caliper (e.g., TOTAL, model TMT322001).
3 Methods
3.1 Germination of M2 Seeds
-
1.
Collect ripe cherries from M1V1 plants in the field and place the fruits in labeled paper bags.
-
2.
Remove the pulp and the mucilage, wash and let dry for 12 days without full sun exposure (see Note 3).
-
3.
Select normal-shaped seeds that are free of visible disease and insects.
-
4.
Treat and cure the seeds with the fungicide Vitavax 40 WP (1 g/Kg) (see Note 4).
-
5.
Label the plastic pots, keeping the same code obtained from their original fruit.
-
6.
Place 10 cm of a substrate in plastic pots and sow the seeds.
-
7.
Add a 1 cm layer of substrate over the sown seeds (approximately 20 and 30 seeds per pot).
-
8.
Place the pots under controlled conditions; humidity greater than 90%; 25–30 °C, with a photoperiod of 12 h.
-
9.
Record the date of planting.
-
10.
Estimate the duration of seed germination and the percentage of germination per progeny.
3.2 Planting Seedlings M2
-
1.
Once the plants have their cotyledonary leaves (see Fig. 1a), transfer the plantlets to polyethylene bags (6 × 8 in) containing substrate (soil, mix of screened compost, and rice husk at 2:1:1).
-
2.
Sow 2 seedlings of the same height and tap root per bag (see Fig. 1b and Note 5).
-
3.
Give a permanent and unique identification code to each plant after planting.
-
4.
Prepare a field map to indicate full details of plant identification and location.
-
5.
Maintain the pots under controlled conditions in a greenhouse or growth chamber with 12 h light LED photoperiod at 28 ± 2 ºC, and (see Fig. 1c).
-
6.
Fertilize with slow-release fertilizer (5 g/plant) (e.g., Osmocote Pro, 19:9:10 + 2MgO + TE) and weekly applications of Bayfolan forte (10 ml/L) to support growth and development.
3.3 Preparation of Coffee Leaf Rust Inoculum
-
1.
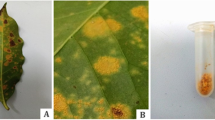
Select coffee leaves with abundant Hemileia vastatrix spores, without the presence of Lecanicillium lecanii (see Fig. 2a).
-
2.
Place the leaves in plastic bags and label (sample number, location, and coffee variety from which it was collected).
-
3.
In the laboratory, using a stereo microscope and a sterile scalpel, scrape the sporulated lesions (only intense orange lesions) (see Fig. 2b, c).
-
4.
Collect spores in sterile 1.5 ml microcentrifuge tubes.
-
5.
Pour 30 ml of distilled water, 0.1 ml of Tween 20, and the uredospores into a sterile tube and shake the suspension of spores (see Fig. 3).
-
6.
Determine the uredospore concentration of the spore suspension using a haemocytometer and a microscope at 10× magnification. Examine five quadrants (4 at the ends and 1 in the center) (see Fig. 3).
-
7.
Count three 50 μl drops of the spore suspension (count only orange-colored spores).
-
8.
Calculate the uredospore density as following: N × 104 × f cell/ml, where “N” is the total counted cells, and “f” is the dilution factor (see Note 6).
-
9.
Adjust inoculum to a concentration of approximately 2.3 × 105 spores/ml.
3.4 Inoculation of the Coffee Leaf Discs with CLR
-
1.
Collect healthy full-grown M1V1 leaves in the greenhouse and keep in a plastic bag on sterilized foam moistened with water (see Note 7).
-
2.
In the laboratory, carefully wash the leaves with water and dry them for 1 h at 24 °C (see Note 8).
-
3.
Clearly mark the humidity chambers (each plastic box) with the plant accession number.
-
4.
Using a 10 mm diameter cylindrical punch, cut out circular leaf discs (see Fig. 4a) without midribs that do not contain stomata (CLR entry point) (Eskes 1982).
-
5.
Ten leaf discs per plant are needed for the detection of resistance. Include susceptible (e.g., C. arabica L. var. Caturra) and resistant plants (e.g., C. canephora, C. arabica var. Obatá) as controls.
-
6.
The discs are moistened and placed upside down in the moist chambers (see Fig. 4b and Note 9).
-
7.
Inoculate each leaf disc with one droplet of approximately 50 μl of the spore suspensions (1 mg spores per mL) (see Fig. 4c).
-
8.
Close the boxes with a transparent lid and keep them at 22.5 ± 1.5 °C in the incubator in the darkness for 36 hours and, a relative humidity greater than 90%, to allow rust germination.
-
9.
After this period, the humidity chambers are uncovered to allow the suspension to dry for 3 h, allowing the evaporation of the inoculation droplets.
-
10.
Incubate at approximately 2,000 lux intensity of artificial light, with 12 h light period and temperature 22.5 ± 1.5° C, with a relative humidity greater than 90% for 15 days.
-
11.
After this period, keep at 23 ± 1.5 °C and relative humidity of 85% under natural light for approximately 10 h and 14 h of darkness for a total of 22 days.
3.5 Evaluation of Plant Resistance Against CLR
-
1.
Record weekly until the appearance of symptoms (chlorotic lesions according to the scale degree 1; see Table 1).
Table 1 Disease severity-rating scale used to record symptoms caused by Hemileia vastatrix in coffee plants (Rozo-Peña and Cristancho-Ardila 2011). -
2.
After observing the first symptoms, the incidence and severity are evaluated every 72 hr for 26 days.
-
3.
Record symptoms and disease severity rate from 29 to 47 days after inoculation on a scale from 0 to 7 using the disease severity rating shown in Table 1 (0 = resistant and 7 = highly susceptible).
-
4.
Calculate disease incidence as follows: [(no. of diseased discs/no. of inoculated discs) * 100] (Rozo-Peña and Cristancho-Ardila 2011).
-
5.
The disease severity is calculated based on the scale shown in Table 1, as follows: [Σ (scale grade * frequency) / (total units observed) * 100] (Rozo-Peña and Cristancho-Ardila 2011; Leguizamón et al. 1998).
-
6.
From infection, calculate the Incubation Period (IP: number of days from inoculation to appearance of chlorosis) and the Latency Period (LP: number of days to appearance of sporulated lesions i.e., uredospores) (Rozo-Peña and Cristancho-Ardila 2011; Leguizamón et al. 1998).
-
7.
Select the plantlets that show CLR resistance (see Note 10).
4 Notes
-
1.
M1V1 coffee plants (Coffea arabica var. Catuaí) were obtained after treatment of embryogenic callus (M0) with the mutagenic agents NaN3 (5 mM for 15 min) and EMS (185.2 mM for 120 min) according to Bolívar-González et al. (2018).
-
2.
M2 coffee seeds of 81 different progenies were obtained after treatment of seeds (M0) with NaN3 (50 mM for 8h) according to Vargas-Segura et al. (2019).
-
3.
Coffee beans rapidly lose their viability when dried (their humidity content cannot be less than 10%).
-
4.
Curing the seed before sowing is the first step to obtain healthy plants; it allows to eliminate pathogens and prevents possible diseases originating from the soil.
-
5.
Young plantlets are highly susceptible to diseases. The best size of the plantlet is 8–10 cm with a tap root of 6–8 cm.
-
6.
To count cells using a haemocytometer or a Neubauer chamber add 15–20 μl of the cell suspension between the haemocytometer and a cover glass. Count the number of cells in all five quadrants (4 at the ends and 1 in the center) and divide by five (see Fig. 3 step 5). The number of cells per square ×105 = the number of spores/ml of the suspension.
-
7.
It is convenient to collect samples of leaves with rust from different locations and coffee varieties to have greater variability of the pathogen.
-
8.
It is recommended to carefully clean the coffee leaves before cutting the discs.
-
9.
The chambers consist of clear sterile plastic boxes (12 × 10 × 3.5 cm) with foam on the bottom. Place a plastic tray with 20 1.2 cm diameter depressions to hold the leaf discs (see Fig. 4b).
-
10.
The procedure proves to be reliable and very sensitive; it is not time-consuming, requiring small amounts of inoculum and plant tissue.
References
Alemayehu D (2017) Review on genetic diversity of coffee (Coffea arabica L.) in Ethiopia. Int J For Hortic (IJFH) 3(2):18–27. https://doi.org/10.20431/2454-9487.0302003
Bolívar-González A, Valdez-Melara M, Gatica-Arias A (2018) Responses of Arabica coffee (Coffea arabica L. var. Catuaí) cell suspensions to chemically induced mutagenesis and salinity stress under in vitro culture conditions. In Vitro Cell Dev Biol Plant 54:576–589. https://doi.org/10.1007/s11627-018-9918-x
Cabral PGC, Maciel-Zambolim E, Oliveira SAS, Caixeta ET, Zambolim L (2016) Genetic diversity and structure of Hemileia vastatrix populations on Coffea spp. Plant Pathol 65(2):196–204. https://doi.org/10.1111/ppa.12411
Dhumal K, Bolbhat S (2012) Induction of genetic variability with gamma radiation and its applications in improvement of horsegram. In: Adrovic F (ed) Gamma radiation. IntechOpen, pp 207–228. https://doi.org/10.5772/37885
Eskes AB (1982) The use of leaf disk inoculations in assessing resistance to coffee leaf rust (Hemileia vastatrix). Neth J Plant Pathol 88(4):127–141. https://doi.org/10.1007/BF01977270
Khan S, Al-Qurainy F, Anwar F (2010) Sodium azide: a chemical mutagen for enhancement of agronomic traits of crop plants. Environ We Int J Sci Technol 4:1–21
Laskar RA, Chaudhary C, Khan S, Chandra A (2018) Induction of mutagenized tomato populations for investigation on agronomic traits and mutant phenotyping. J Saudi Soc Agric Sci 17(1):51–60. https://doi.org/10.1016/J.JSSAS.2016.01.002
Leguizamón J, Orozco L, Gómez L (1998) Períodos de incubación (pi) y de latencia (pl) de la roya del cafeto en la zona cafetera central de Colombia. Cenicafé 49(56)
Lima JD, Maigret B, Fernandez D, Decloquement J, Pinho D, Albuquerque EVVS, Rodrigues MO, Martins NF (2020) Searching in silico novel targets for specific coffee rust disease control. In: Kowada L, de Oliveira D (eds) Advances in bioinformatics and computational biology. BSB 2019. Lecture notes in computer science, vol 11347. Springer, Cham. https://doi.org/10.1007/978-3-030-46417-2_10
Mago R, Till B, Periyannan S, Yu G, Wulff BBH, Lagudah E (2017) Generation of loss-of-function mutants for wheat rust disease resistance gene cloning. In: Periyannan S (ed) Wheat rust diseases: methods in molecular biology, vol 1659. Humana Press, New York. https://doi.org/10.1007/978-1-4939-7249-4_17
McCook S (2006) Global rust belt: Hemileia vastatrix and the ecological integration of world coffee production since 1850. J Glob Hist 1(2):177–195. https://doi.org/10.1017/S174002280600012X
Melese Ashebre K (2016) The role of biotechnology on coffee plant propagation: a current topics paper. J Biol Agric Healthc 6(5). www.iiste.org
Mishra MK, Slater A (2012) Recent advances in the genetic transformation of coffee. Biotechnol Res Int 2012:1–17. https://doi.org/10.1155/2012/580857
Rozo-Peña YI, Cristancho-Ardila M (2011) Evaluación de la susceptibilidad de Hemileia vastatrix Berk and Br a fungicidas del grupo de los triazoles. Cenicafé 61(4)
Talhinhas P, Batista D, Diniz I, Vieira A, Silva D, Loureiro A, do Céu Silva M (2017) The coffee leaf rust pathogen Hemileia vastatrix: one and a half centuries around the tropics. Mol Plant Pathol 18(8):1039–1051. https://doi.org/10.1111/mpp.12512
Vargas-Segura C, López-Gamboa E, Araya-Valverde E, Valdez-Melara, M., Gatica-Arias A (2019) Sensitivity of seeds to chemical mutagens, detection of DNA polymorphisms and agro-metrical traits in M1 generation of coffee (Coffea arabica L.). J Crop Sci Biotechnol 22(5):451–464. https://doi.org/10.1007/S12892-019-0175-0
Waller JM, Bigger M, Hillocks RJ (2007) Coffee pests, diseases and their management, pp 1–434. https://doi.org/10.1079/9781845931292.0000
Zambolim L (2016) Current status and management of coffee leaf rust in Brazil. Trop Plant Pathol 41(1):1–8. https://doi.org/10.1007/S40858-016-0065-9
Acknowledgments
This work was funded by the Consejo Nacional de Rectores (CONARE), project “Evaluation of alternative sources of genetic resistance to coffee rust (Hemileia vastatrix)”, (project No. 5401-1701-6140). A-Gatica-Arias acknowledged the Cátedra Humboldt 2023 of the University of Costa Rica for supporting the dissemination of biotechnology for the conservation and sustainable use of biodiversity.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Rojas-Chacón, J.A., Echeverría-Beirute, F., Gatica-Arias, A. (2023). Evaluation of Coffee (Coffea arabica L. var. Catuaí) Tolerance to Leaf Rust (Hemileia vastatrix) Using Inoculation of Leaf Discs Under Controlled Conditions. In: Ingelbrecht, I.L., Silva, M.d.C.L.d., Jankowicz-Cieslak, J. (eds) Mutation Breeding in Coffee with Special Reference to Leaf Rust. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-67273-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-662-67273-0_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-67272-3
Online ISBN: 978-3-662-67273-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)