Abstract
The coffee leaf rust, a disease caused by the biotrophic fungus Hemileia vastatrix, is one of the main limitations in coffee production today as it causes significant economic losses to the coffee production sector. Genetic improvement is an option to solve these problems. The Arabica varieties have a very narrow genetic base therefore the induction of mutations, through e.g. physical methods such as gamma rays, could be an efficient tool to increase the genetic diversity of the crop. This would allow to obtain desirable agronomic characteristics such as resistance to pests and diseases. To determine the effect of irradiation on the plants, protocols enabling evaluation of improved traits must be applied. In the case of the assessment of plant resistance to pests and diseases, screening protocols that take into account their biology should be considered. This chapter provides a detailed protocol for the inoculation and evaluation of Hemileia vastatrix under laboratory conditions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Coffee is the second most commercialized product worldwide. It is produced in over 50 countries and secures livelihoods for millions of farmers (Vega et al. 2003; ICAFE 2017). Hemileia vastatrix Berk. & Broome, the causal agent of coffee leaf rust, is one of the biotic factors that affects coffee, causing significant economic losses due to the defoliation, subsequent harvest losses, and renewal needs due to severe damage caused in plants (Barquero 2013; ICAFE 2013).
This disease caused a widespread impact in 2012 in Central America, mainly due to the susceptibility of planted varieties of Coffea arabica such as Caturra and Catuaí (Avelino and Rivas 2013). Due to the origin, domestication process, reproduction and evolution of the genome, Arabica varieties are characterized by a low genetic diversity (Hendre et al. 2008; Prakash et al. 2002). As a result of a natural hybridization process between C. eugenioides and C. canephora, C. arabica is the only tetraploid species of the genus Coffea (2n = 4x = 44). This generates a limitation for genetic improvement of resistance genes due to the homogeneity of the varieties (Jefuka et al. 2010; Naranjo Zúñiga 2018).
Hemileia vastatrix is a biotrophic fungus that penetrates the plant through the stomata located on the abaxial side of the leaf. Its taxonomic classification is as follows: Phylum: Basidiomycota, Class:Pucciniomycetes, Order: Pucciniales, Family: Zaghouaniaceae, Genus: Hemileia (gbif 2022).
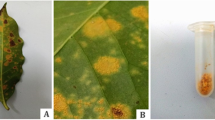
H. vastatrix is a hemicyclic fungus producing urediniospores, teliospores and basidiospores, but only the dikaryotic urediniospores, which form the asexual part of the cycle, reinfect coffee leaves successively and are responsible for the disease (as revised by Talhinhas et al. 2017). As a first symptom, small yellow chlorotic spots are observed in the foliage that subsequently, as the infection progresses, produce masses of orange urediniosporic sori (see Fig. 1) (Arauz Cavallini 2011). An epidemic of coffee leaf rust can be divided into two stages: stage of the production of the initial inoculum, whose main source is the residual inoculum, and a second stage that comprises the production of the secondary inoculum, which is the result of the successive repetition of the infection process on the same leaf (Avelino and Rivas 2013, Naranjo Zúñiga 2018).
The disease cycle of coffee leaf rust consists of five stages which can be affected by factors such as fruit load, plant resistance, microclimate and plant nutrition (Avelino 2004; Rhiney et al. 2021).
These stages include:
-
Dissemination: It occurs in three stages, the release of urediniospores, dispersion through factors such as rain, wind and people and deposition in plant tissue.
-
Germination: Once the urediniospores are deposited in the leaves, 4–6 germinative tubes are emitted with an appressorium necessary to force stomatal entry. The optimal conditions are 22 ºC temperature, 24 h of darkness and free water until the penetration stage (Silva et al. 1999; Naranjo Zúñiga 2018).
-
Penetration: The presence of well-formed stomata is necessary to be able to enter the leaf, so that the age of the leaf influences the receptivity to infection.
-
Colonization: This phase requires the growth of hyphae of the fungus in the intercellular spaces of the spongy parenchyma and haustoria within the cells of the palisade parenchyma and even the upper epidermis to give rise to the first symptoms, a macroscopic chlorosis (McCain and Hennen, 1984).
-
Sporulation: Once hyphae invade the substomatal chamber, they differentiate to form protosori. Later urediniosporic sori protrude through the stomata (Silva et al. 1999 and references therein).
Genetic improvement is an attractive approach that enables solving production constraints caused by pests and diseases. Induced mutagenesis is one of the tools that can be used to increase genetic diversity (ICAFE 2011; Novak and Brunner 1992; Shu et al. 2011). Gamma rays have proven to be an efficient tool to improve traits of agronomic importance such as resistance to pests and diseases (Borzouei et al. 2010; Yadav and Singh 2013; Shu et al. 2011). An efficient screening protocol is therefore required for evaluation of mutant populations developed via induced mutagenesis.
This protocol describes the procedures for the inoculation and evaluation of the defense response of the coffee genetic material infected with Hemileia vastatrix under laboratory conditions, or the biological efficacy of molecules for the control of the disease. This protocol is based on Eskes and Toma-Braghini (1982), with modifications made by the Costa Rican Coffee Institute-Coffee Research Center, Phytoprotection Laboratory.
2 Materials
2.1 Preparation of Rust Inoculum
-
1.
Sterile scalpel.
-
2.
50 µL centrifuge tubes.
-
3.
Falcon tubes of the necessary size.
-
4.
Distilled water.
-
5.
Neubauer chamber.
-
6.
Microscope.
2.2 Rust Inoculation
-
1.
Scissors.
-
2.
Plastic boxes.
-
3.
Foams.
-
4.
Plastic grid.
-
5.
Water.
-
6.
Micropipette (50 µl graduation).
-
7.
Micropipette tips.
-
8.
Ceramic spoon.
-
9.
Adhesive plastic.
2.3 Rust Evaluation
-
1.
Magnifying glass.
-
2.
Light source.
3 Methods
3.1 Preparation of Rust Inocula
-
1.
Collect coffee leaves with abundant rust spores (race II, or the most important for the region or country) from the field or greenhouse-grown susceptible varieties (Caturra or Villa Sarchí) (see Note 1).
-
2.
Scrape the spores with a sterile scalpel and store in 50 µL centrifuge tubes.
-
3.
Prepare a suspension of spores in distilled water and determine the concentration of urediniospores using a Neubauer chamber. Count the spores with orange coloration located in the corners and the center of the central sub-chamber (see Fig. 2).
-
4.
Determine the concentration of urediniospores applying the formula (see Note 2):
$$Urediniospore\,concentration/ml = urediniospores\,counted\, \times 5 \times 1e10^{4}$$
3.2 Rust Inoculation
-
1.
Collect healthy leaves from the second node of branches from the middle stratum of the plants selected for rust resistance evaluation (see Fig. 3 and Note 3).
-
2.
Cut 2 × 2 cm square segments from collected leaves.
-
3.
Prepare humid chambers that consist of a plastic box with a wet foam in the background and a plastic grid on the foam.
-
4.
Place the leaf segments on the foam and inoculate with 50 µl of suspension by placing a drop in the center and spreading it with a ceramic spoon.
-
5.
Cover the wet chamber with clear adhesive plastic, ensuring it is airtight.
-
6.
Incubate in darkness at room temperature for 3 days (see Note 4).
-
7.
Transfer into a room with a photoperiod of 12 h light /12 h dark and 22 ± 1 °C for 28 days.
3.3 Rust Evaluation
-
1.
Uncover the humid chambers and place in a location with enough light.
-
2.
Remove necrotic segments.
-
3.
Count number of segments inoculated and number of segments with rust.
-
4.
The incidence rate of the disease is determined by the formula:
$$\frac{{\% \,{\text{incidence}} = {\text{Number of segments with presence of uredospores}}\, \times {1}00}}{{\text{total segments inoculated}}}$$
-
5.
Determine the presence and abundance of signs and symptoms (Table 1).
Table 1 Scale used to measure the severity of rust in coffee segments (Eskes and Toma-Braghini 1982) (see Note 5)
This inoculation technique can also be used to evaluate the response of different natural or chemically synthesized molecules for the defense of plants susceptible to the disease. To do this, the use of Table 1 allows us to understand the mechanism of action of the products according to the incubation and latency periods of the pathogen.
4 Notes
-
1.
When selecting leaves to collect rust spores, it is necessary to check whether the fungus Lecanicillium lecanii, a hyperparasite of H. vastatrix, is not present. Application of fungicides should be avoided two months prior to collection of leaves.
-
2.
The concentration of the spore suspension should be approximately 1 × 105 urediniospores/ml.
-
3.
It may be important to evaluate different leaf ages of individual mutant plants with putative resistance to the disease.
-
4.
Some protocols (in greenhouse and laboratory conditions) indicate that 24 h of incubation is enough. At this time, the fungus concludes the germination, appressoria differentiation, and penetrates the host tissues. In our laboratory, the sporulation is more abundant and successful when dark conditions remain for three days. Therefore, it is recommended that this step is being tested and adjusted to the conditions of the laboratory in which the evaluation is going to be performed.
-
5.
In this protocol, in addition to the rust incidence the scale described in Table1 is being used. The inoculation technique presented in this chapter can also be used to evaluate the response of different natural or chemically synthesized molecules for the defense of plants susceptible to the disease. To do this, the use of Table 1 allows us to understand the mechanism of action of the products according to the incubation and latency periods of the pathogen.
References
Arauz Cavallini LF (2011) Fitopatología: un enfoque agroecológico, 2 edn. UCR, San José, Costa Rica, 514 p
Avelino et al (2004) Effects of crop management patterns on coffee rust epidemics. Plant Pathol 53:541–547. https://doi.org/10.1111/j.1365-3059.2004.01067.x
Avelino J, Rivas G (2013) La roya anaranjada del cafeto. hal-01071036, pp 1–47. Available in https://hal.archives-ouvertes.fr/hal-01071036 Accessed 19 Nov 2019
Barquero M (2013) Recomendaciones para el combate de la roya del cafeto, 3 edn. Heredia, CR, ICAFE, 72 p. Available in https://www.researchgate.net/publication/281625030_Recomendaciones_para_el_combate_de_la_roya_del_cafeto
Borzouei A, Kafi M, Khazaei H, Naseriyan B, Majdabadi A (2010) Effects of gamma radiation on germination and physiological aspects of wheat (Triticum aestivum L.) seedlings. Pakistan J Botany 42(4):2281–2290
Eskes AB, Toma-Braghini M (1982) The effect of leaf age on incomplete resistance of coffee to Hemileia vastatrix. Netherlands J Plant Pathol 88(6):219–230. https://doi.org/10.1007/BF02000128Gbif. Global Biodiversity Information Facility—https://www.gbif.org/search?q=Hemileia%20vastatrix. Accessed 21 Dec 2022
GBIF (2022) Gobal Biodiversity Information Facility. www.gbig.org
Hendre PS, Phanindranath R, Annapurna V, Lalremruata A, Aggarwal RK (2008) Development of new genomic microsatellite markers from robusta coffee (Coffea canephora Pierre ex A. Froehner) showing broad cross-species transferability and utility in genetic studies. BMC Plant Biol 8(1):51
ICAFE (2011) Guía técnica para el cultivo del café. 1 ed. Heredia, CR, ICAFE, 72 p
ICAFE (2013) Informe sobre la actividad cafetalera de Costa Rica. Heredia, CR, ICAFE, 69 p
ICAFE (2017) Informe sobre la actividad cafetalera de Costa Rica. Heredia, CR, ICAFE, 59 p
Jefuka C, Fininsa C, Adugna G, Hindort H (2010) Coffee leaf rust epidemics (Hemileia vastatrix) in Montane Coffee (Coffea arabica L.) Forests in Southwestern Ethiopia. East. Afr J Sci 4(2):86–95
Naranjo Zúñiga VR (2018) Evaluación del efecto de diferentes manejos de nutrición y sombra sobre la resistencia fisiológica de la planta de café (Coffea arabica) a la roya (Hemileia vastatrix), en discos de hoja en condiciones controladas de laboratorio (Doctoral dissertation, CATIE, Turrialba, Costa Rica), 68 p
Novak FJ, Brunner H (1992) Tecnología de mutación inducida para el mejoramiento genético de los cultivos. IAEA Newsletter 4:25–33
McCain JW, Hennen JF (1984) Development of the uredinial thallus and sorus in the orange coffee rust fungus, Hemileia vastatrix. Phytopathology 74:714–721
Prakash NS, Combes MC, Somanna N, Lashermes P (2002) AFLP analysis of introgression in coffee cultivars (Coffea arabica L.) derived from a natural interspecific hybrid. Euphytica 124:265–271
Rivillas Osorio CA, Serna Giraldo CA, Cristancho Ardila MA, Gaitan Bustamante AL (2011) La roya del cafeto en Colombia—Impacto, manejo y costos del control. Caldas, Colombia, Cenicafé, 51 p
Rhiney K, Guido Z, Knudson C, Avelino J, Bacon CM, Leclerc G, Aime MC, Bebber DP (2021) Epidemics and the future of coffee production. Proc Natl Acad Sci USA 118:e2023212118
Shu QY, Forster BP, Nakagawa H (2011) Plant mutation breeding and biotechonology. FAO/IAEA, Viena, 589 p
Silva MC, Nicole M, Rijo L, Geiger JP, Rodrigues CJ (1999) Cytochemistry of plant-rust fungus interface during the compatible interaction Coffea arabica (cv. Caturra)-Hemileia vastatrix (race III). Int J Plant Sci 160:79–91
Talhinhas P, Batista D, Diniz I, Vieira A, Silva DN, Loureiro A, Tavares S, Pereira AP, Azinheira HG, Guerra-Guimarães L, Várzea V, Silva MC (2017) The Coffee Leaf Rust pathogen Hemileia vastatrix: one and a half centuries around the tropics. Mol Plant Pathol 18:1039–1051. https://doi.org/10.1111/mpp.12512
Vega FE, Rosenquist E, Collins W (2003) Global project needed to tackle coffee crisis. Nature 425:343
Yadav A, Singh B (2013) Effects of gamma irradiation on germination and physiological aspect of maize genotypes. Int J Biotechnol Bioeng Res 4(6):519–520
Acknowledgements
Funding for this work was provided by the Costa Rican Coffee Institute-Coffee Research Center and the FAO/IAEA Joint Center. This work is part of the Coordinated Research Project D22005 titled “Efficient Screening Techniques to Identify Mutants with Disease Resistance for Coffee and Banana”, Contract Number 20475.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Barquero-Miranda, M., Cordero-Vega, M.J., Ureña-Ureña, K. (2023). Inoculation and Evaluation of Hemileia vastatrix Under Laboratory Conditions. In: Ingelbrecht, I.L., Silva, M.d.C.L.d., Jankowicz-Cieslak, J. (eds) Mutation Breeding in Coffee with Special Reference to Leaf Rust. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-67273-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-662-67273-0_16
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-67272-3
Online ISBN: 978-3-662-67273-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)