Abstract
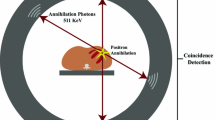
Positron emission tomography (PET) is a non-invasive imaging technique that employs positron-emitting radionuclides labelled to biological molecules. Unlike other imaging techniques, such as computer tomography (CT) and magnetic resonance imaging (MRI) that provide anatomical or structural information, PET allows obtaining unique quantitative information of important biologic processes in vivo (e.g. myocardial perfusion and metabolism, inflammation, innervation, receptor density).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Positron emission tomography (PET) is a non-invasive imaging technique that employs positron-emitting radionuclides labelled to biological molecules. Unlike other imaging techniques, such as computer tomography (CT) and magnetic resonance imaging (MRI) that provide anatomical or structural information, PET allows obtaining unique quantitative information of important biologic processes in vivo (e.g. myocardial perfusion and metabolism, inflammation, innervation, receptor density).
Although there is expanded use of PET for oncology, cardiac PET is emerging as an important modality for the detection of physiologically significant coronary artery disease (CAD), evaluation of infiltrative diseases (e.g. sarcoidosis, amyloidosis), assessment of myocardial viability, and for infective endocarditis. Modern PET systems are combined with CT, which provides additional information about the burden of atherosclerosis and plaque morphology.
A unique advantage of PET is its ability to enable absolute quantification of myocardial blood flow in mL/min/g of myocardial tissue. As reviewed in this Atlas, the quantitative blood flow information enhances the diagnostic value of PET myocardial perfusion imaging, improves risk stratification and helps guide patient management.
1.1 General Description of PET Radiopharmaceuticals
PET Radiotracers for Myocardial Perfusion Imaging
Rubidium-82 (Rb-82): It is a monovalent cationic analogue of potassium and is produced in a commercially available generator by decay from strontium-82 attached to an elution column. It is the most commonly used radiopharmaceutical for myocardial perfusion imaging with PET, particularly in the USA. The strontium-82 has a half-life of 25.5 days and decays to rubidium-82 by electron capture. The physical half-life of Rb-82 is 76 seconds. Rb-82 is eluted with normal saline by a computer-controlled elution pump, directly connected by intravenous tubing to the patient. The generator can be eluted approximately every 10 min, which allows for very rapid serial rest and stress imaging. Given its ultra-short half-life, exercise is not possible with Rb-82. Rb-82 is extracted from plasma with high efficiency by myocardial cells via the Na+/K+ ATPase pump. Myocardial extraction of Rb-82 is superior to that of Tc99m-labelled perfusion tracers but lower than that of N-13 ammonia at high flow rates. The energy of positrons emitted from the decay of Rb-82 is much higher than that of N-13 or F-18. Consequently, the distance between the decay site and the annihilation site (so-called positron range) is higher for Rb-82, which negatively affects the spatial resolution of PET images.
N-13 Ammonia: It has a high first pass extraction at high myocardial blood flow, which makes it ideal for myocardial perfusion imaging. The main limitation is that its 9.96-min half-life requires an on-site cyclotron and radiochemistry synthesis capability. Novel ‘bench top’ cyclotrons have recently become commercially available potentially allowing more widespread clinical use of N-13 ammonia. This commercially available mini-cyclotron with automated synthesis allows on-site production of N-13 ammonia without the need of a larger production facility. Following IV injection, it undergoes rapid blood pool clearance with diffusion across cell membranes and trapping inside the cardiomyocyte following irreversible enzymatic conversion to glutamic acid. Myocardial retention of N-13 ammonia may be heterogeneous in some patients with apparent defects in the lateral left ventricular wall. N-13 ammonia images also may be degraded by occasional intense liver activity, which can interfere with the evaluation of the inferior wall. Although the sequestration of N-13 ammonia in the lungs is usually minimal, it may be increased in patients with depressed left ventricular systolic function or chronic pulmonary disease and, occasionally, in smokers and interfere with the evaluation of the lateral wall. Its relatively long half-life also allows to be combined with exercise.
O-15 Water: It is a cyclotron product and has a physical half-life of 2.07 min. O-15 water is a freely diffusible agent with very high myocardial extraction across a wide range of myocardial blood flows. The degree of extraction is independent of flow which makes it an ideal agent for quantification of myocardial blood flow. However, because it is a freely diffusible tracer, imaging is challenging due to its high concentration in the blood pool. Parametric flow images can be used to delineate the presence and extent of regional perfusion defects, but they are of relatively lower quality compared to Rb-82 and N-13 ammonia. Generation of gated images for calculation of LV volumes and ejection fraction is challenging and not performed routinely.
F-18 Flurpiridaz: It is an investigational perfusion tracer currently under evaluation in phase III clinical trials (18F-BMS747158-02, NCT03354273). It has higher first pass myocardial extraction than Rb-82 and N-13 ammonia. The use of F-18 with a 108-min half-life makes it ideal for unit dose distribution, thereby facilitating broader access to cardiac PET imaging without the need of an on-site cyclotron or a 82St/82Rb generator.
Myocardial Metabolism
F-18 Fluorodeoxyglucose (FDG): It is an analogue of glucose produced in a cyclotron with associated specialized radiochemistry modules. The relatively long half-life of F-18 allows for off-site production and commercial unit dose distribution. Like regular glucose, FDG is transported into the myocardium by specific glucose transporters (GLUT-1 and GLUT-4) via facilitated diffusion. Inside the cardiomyocyte, FDG undergoes phosphorylation and trapping and is a marker of glucose metabolism. FDG is currently used for myocardial viability assessment and for delineation of myocardial inflammation/infection.
1.2 PET Acquisition Protocols
Figure 1.1 below provides a schematic representation of the common protocols used for imaging myocardial perfusion with PET/CT. Please see specific protocols for myocardial viability in Sect. 2.7 and inflammation/infection imaging in Sect. 4.1.
CT Scan: Patient positioning is performed with a CT scout image or topogram, which is followed by a low-dose CT transmission scan used for correction of attenuation by soft tissues. The acquisition parameters for the CT transmission scan varies with the configuration of the CT scanner (e.g. 8, 16, 64 multidetector CT). However, the general scan settings include a slow rotation speed, relatively high pitch, variable tube potential (e.g. 80–140 kVp depending on patient size) and a low tube current. The CT transmission scan is non-gated and acquired during shallow free breathing. In patients without known CAD, it is common to also include a separate gated CT scan for quantification of coronary artery calcifications. In the absence of a non-contrast gated CT scan, coronary artery calcifications can also be assessed semi-quantitatively from the CT transmission scan obtained for attenuation correction. In selected patients, it is also possible to obtain a coronary CT angiogram (CCTA) immediately following the assessment of myocardial perfusion, but this necessitates at least a 64-slice multidetector CT scanner.
Emission Scans: The radiotracer dose should be adjusted based on the patient size, equipment and acquisition protocol (i.e. 2D vs 3D mode), and imaging protocol (e.g. same dose vs low-high dose protocols for N-13 ammonia). There are several ways in which the emission perfusion images can be acquired including:
-
ECG Gated imaging: It is the most common clinical approach when using scanners without list-mode acquisition capability. Imaging begins 90–120 s after radiotracer to allow for clearance of radioactivity from the lungs and blood pool. Scan duration depends on type of equipment (e.g. analogue vs digital PET camera) and radiotracer used (e.g. ~7 min for rubidium-82 and 10–15 min for N-13 ammonia). The number of gated frames is usually set to 8 or 16.
-
Multi-frame or dynamic imaging: It consists of a series of multiple static images with a pre-determined time frames. Imaging begins with the radiotracer bolus and extends for 7–15 min depending on the radiotracer used, as described above. This acquisition mode is essential for quantification of myocardial blood flow (in mL/min/g).
-
List-mode imaging: This is the ideal and most common approach when using modern PET cameras. In a list-mode acquisition, each coincidence event is recorded with detection time and position information, as well as ECG information that allows to determine the time the event occurred during the cardiac cycle. The detection time information is used to retrospectively format the data into multiple time frames after completion of the acquisition. List-mode data can be then reformatted in many different ways including static or summed images, gated images and multi-frame or dynamic images.
Stress Testing: It is usually performed with pharmacologic means, most commonly vasodilators (e.g. adenosine, dipyridamole, or regadenoson) or alternatively inotropes (i.e. dobutamine). As briefly mentioned in Sect. 1.2, exercise can be performed with N-13 ammonia and in the future it will also be possible with F-18 flurpiridaz. Exercise cannot be performed with rubidium-82 or O-15 water. It is important to keep in mind that exercise protocols do not currently allow to quantify myocardial blood flow, which as described above necessitates the acquisition of the initial arterial phase data to generate an arterial input function.
Myocardial Perfusion Imaging Sequence
-
Rest-stress. This is the most common sequence. Given the ultra-short half-life of Rb-82 and O-15 water, approximately the same dose is used for both rest and stress imaging without the need to wait for decay of the initial dose before administering the stress dose. With N-13 ammonia, the same dose for both rest and stress imaging is most common. This requires a slightly longer wait between rest and stress imaging to allow for radioactive decay of the initial dose (~20–30 min). With modern PET cameras, it is possible to perform low-dose rest-high dose stress imaging without a wait, which shortens the protocol substantially (approximately 35 min).
-
Stress-rest. Some laboratories perform stress imaging first, as a normal scan may avoid the need for rest imaging. The downside of ‘stress-only’ imaging is that there will be no opportunity to obtain rest and stress LV ejection fraction (EF) or myocardial blood flow reserve, which enhance the diagnosis, risk stratification and patient management as described in the case-based discussions.
-
Stress-only. Please see limitations above. Perhaps this protocol is ideally suited for patients undergoing exercise stress PET myocardial perfusion imaging.
Further Reading
-
Murthy, V. L., Bateman, T. M., Beanlands, R. S., Berman, D. S., Borges-Neto, S., Chareonthaitawee, P. Corbett, J. R. (2017). Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. Journal of Nuclear Cardiology, 25(1), 269–297.
-
Dilsizian, V., Bacharach, S.L., Beanlands, R.S. et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. Journal of Nuclear Cardiology (2016); 23: 1187–1226.
1.3 Examples of Studies with 13N-Ammonia; 82Rubidium and 15O-Water
Case 1: PET MPI Using 13N-Ammonia
History
-
A 59-year-old male with hypertension, dyslipidaemia, diabetes mellitus and obesity without known coronary artery disease (CAD) was referred for a rest/stress 13N-ammonia myocardial perfusion PET/CT to evaluate for atypical angina and dyspnoea (Figs. 1.2 and 1.3).
PET/CT Images
Rest and Regadenoson-stress myocardial perfusion images obtained with 13N-ammonia as the perfusion tracer. Images are displayed in short axis (top), horizontal long axis (middle) and vertical long axis (bottom) of the heart with the stress images on the top of each pair. The images demonstrate normal regional myocardial perfusion. The isolated small defect on the infero-apical segment is often seen, reflects a partial volume effect and does not represent a pathological finding
TOP panel: Time-activity curves for the arterial input function (dark and light green curves) and myocardial tissue (yellow and red curves) for the stress and rest myocardial perfusion images. BOTTOM panel: Myocardial blood flow (MBF) measurements (in mL/min/g) at rest and during Regadenoson-stress for each coronary artery territory and for the entire left ventricle. The myocardial flow reserve (stress over rest MBF) is also shown. The results show normal maximal MBF (>1.8 mL/min/g) and flow reserve (>2) in all coronary territories and for the entire left ventricle
Findings
-
The rest and stress images demonstrate normal myocardial perfusion.
-
The quantitative myocardial blood flow and flow reserve are normal both regionally and globally.
-
LVEF at rest was 59% and increased to 64% post stress. LV volumes were normal (values not shown).
Differential Diagnosis
-
Obstructive CAD
-
Coronary microvascular dysfunction
Correlative Imaging
-
None
Management
-
Reassurance and risk factor management
Teaching Points
-
A visually normal myocardial perfusion PET study with normal stress myocardial blood flow and flow reserve has very high sensitivity and negative predictive value for excluding flow limiting CAD.
-
The normal myocardial flow reserve excludes the possibility of obstructive CAD and coronary microvascular dysfunction.
Further Reading
-
Murthy V, Bateman T, Beanlands R, Berman D, Borges-Neto S, Chareonthaitawee P, et al. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. Journal of Nuclear Cardiology. 2017;25:269–297.
-
Ziadi M, deKemp R, Williams K, Guo A, Renaud J, Chow B, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? Journal of Nuclear Cardiology. 2012;19:670–680.
-
Naya M, Murthy V, Taqueti V, Foster C, Klein J, Garber M, et al. Preserved Coronary Flow Reserve Effectively Excludes High-Risk Coronary Artery Disease on Angiography. Journal of Nuclear Medicine. 2014;55:248–255.
Case 2: PET MPI Using 82Rubidium
History
-
A 72-year-old male with exertional dyspnoea
-
History of hypertension, diabetes mellitus and obesity. He was referred for a rest/stress myocardial perfusion PET/CT to evaluate possible flow limiting CAD
Findings
-
The rest and stress myocardial perfusion images demonstrate no evidence of regional perfusion abnormalities (Fig. 1.4).
-
The quantitative myocardial blood flow and flow reserve (MFR: normal values >2) are normal both regionally and globally (Fig. 1.5).
Rest and Regadenoson-stress myocardial perfusion images obtained with 82Rubidium as the perfusion tracer. Images are displayed in short axis (top), horizontal long axis (middle) and vertical long axis (bottom) of the heart with the stress images on the top of each pair. The images demonstrate normal and homogeneous distribution of the radiotracer throughout the LV without regional perfusion defects
Myocardial blood flow (MBF) measurements (in mL/min/g) at rest and during Regadenoson-stress for each coronary artery territory and for the entire left ventricle are shown in Fig. 1.3. The myocardial flow reserve (stress over rest MBF) is also shown.
Differential Diagnosis
-
None
Correlative Imaging
-
None
Management
-
Reassurance and risk factor management
Teaching Points
-
As discussed in Case # 1 with 13N-ammonia, a visually normal myocardial perfusion 82Rubidium PET study with normal stress myocardial blood flow and flow reserve has very high sensitivity and negative predictive value for excluding flow limiting CAD.
-
The normal myocardial flow reserve excludes the possibility of coronary microvascular dysfunction.
Further Reading
-
Mc Ardle B, Dowsley T, deKemp R, Wells G, Beanlands R. Does Rubidium-82 PET Have Superior Accuracy to SPECT Perfusion Imaging for the Diagnosis of Obstructive Coronary Disease?. Journal of the American College of Cardiology. 2012;60:1828–1837.
-
Parker M, Iskandar A, Limone B, Perugini A, Kim H, Jones C, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circulation Cardiovascular Imaging. 2020;5:700–707.
-
Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguadé-Bruix S, et al. Detection of Significant Coronary Artery Disease by Noninvasive Anatomical and Functional Imaging. Circulation: Cardiovascular Imaging. 2015;8.
-
Takx R, Blomberg B, Aidi H, Habets J, de Jong P, Nagel E, et al. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography With Fractional Flow Reserve Meta-Analysis. Circulation: Cardiovascular Imaging. 2015;8. https://doi.org/10.1161/CIRCIMAGING.114.002666
Acknowledgement
82Rubidium images are courtesy of Dr. Mouaz Al-Mallah, Methodist Hospital, Houston, Texas.
Case 3: PET MPI Using 15O-Water
History
-
A 59-year-old female with a history of hyperlipidaemia, hypertension, obesity and hyperthyroidism was referred for a rest/stress 15O-water myocardial perfusion PET/CT to evaluate for atypical angina and fatigue (Figs. 1.6 and 1.7).
PET/CT Images
Rest and Regadenoson-stress myocardial perfusion images obtained with 15O-water as the perfusion tracer. Images are displayed in short axis (top), horizontal long axis (middle) and vertical long axis (bottom) of the heart with the stress images on the top of each pair. The images demonstrate normal and homogeneous distribution of the radiotracer throughout the LV without regional perfusion defects
TOP panel: parametric polar maps depicting segmental MBF values for the vasodilator-stress and rest 15O-water myocardial perfusion images. The colour in the polar maps is scaled to the corresponding MBF value. The polar map on the far right depicts the corresponding myocardial flow reserve. BOTTOM panel: shows the corresponding rest and stress MBF values, and the flow reserve
Findings
-
The rest and stress parametric blood flow images demonstrate normal myocardial perfusion. The parametric images are scaled to the maximum blood flow value, hence the difference relative intensity on the stress and rest images.
-
The quantitative myocardial blood flow and flow reserve are normal both regionally and globally.
Differential Diagnosis
-
Obstructive CAD
-
Coronary microvascular dysfunction
Correlative Imaging
-
None
Management
-
Reassurance and risk factor management
Teaching Points
-
A visually normal myocardial perfusion PET study with normal stress myocardial blood flow and flow reserve has very high sensitivity and negative predictive value for excluding flow limiting CAD. Indeed, a recent comparative effectiveness trial demonstrated that quantitative 15O-water myocardial perfusion PET had the highest accuracy for diagnosing and ruling out obstructive CAD compared to CT coronary angiography with or without FFRCT and SPECT myocardial perfusion imaging (see suggested reading).
-
The normal myocardial flow reserve also excludes the possibility of coronary microvascular dysfunction.
Further Reading
-
Danad I, Raijmakers P, Driessen R, Leipsic J, Raju R, Naoum C, et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiology. 2017;2:1100–1107.
-
Driessen R, Danad I, Stuijfzand W, Raijmakers P, Schumacher S, van Diemen P, et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. Journal of the American College of Cardiology. 2019;73:161–173.
Acknowledgement
15O-water images are courtesy of Drs. Henrich Harms and Jens Sorensen, Uppsala University, Sweden.
1.4 Recognizing and Troubleshooting Artefacts
1.4.1 Misregistration
Case 4
History
-
A 57-year-old male without a history of coronary artery disease (CAD) was referred for a rest/stress myocardial perfusion PET/CT to evaluate for atypical angina (Figs. 1.8 and 1.9).
PET/CT Images
LEFT panel: Rest and Regadenoson-stress myocardial perfusion PET/CT images obtained with 13N-ammonia. The display is as in case # 1. The images demonstrate a medium sized perfusion defect of severe intensity involving the mid and basal anterolateral and inferolateral LV segments (arrows), showing complete reversibility. RIGHT panel: The fused perfusion/CT transmission images demonstrate misregistration of the lateral and anterolateral walls on the stress images, which are overlapping the left lung field on the CT (arrows). This misalignment leads to an attenuation correction artefact, resulting in an apparent perfusion defect
Findings
-
The original reconstructed stress images demonstrate a medium sized perfusion defect of severe intensity involving the anterolateral wall (arrows), showing complete reversibility.
-
However, inspection of the fused emission/CT transmission images demonstrate misalignment between the two datasets with the anterolateral wall overlapping the lung field on the CT images.
-
Correction of the emission/CT transmission misalignment resulted in resolution of the perfusion defect and a normal scan.
Differential Diagnosis
-
Obstructive CAD with single vessel myocardial ischaemia
Correlative Imaging
-
None
Management
-
Reassurance and risk factor management
Teaching Points
-
Careful inspection of the emission/CT transmission alignment is a critical quality control step in the assessment of cardiac PET/CT images.
-
Identification of misalignment should be corrected and images re-reconstructed with the proper alignment before clinical interpretation.
-
The perfusion defect is caused from incorrectly applying attenuation coefficients corresponding to lung tissue (air), resulting in under-correction of attenuation in the misregistered region that appears cold compared to the rest of the heart corrected with soft tissue attenuation coefficients.
Further Reading
-
Bettinardi V, Gilardi MC, Lucignani G, Landoni C, Rizzo G, Striano G, et al. A procedure for patient repositioning and compensation for misalignment between transmission and emission data in PET heart studies. Journal of Nuclear Medicine. 1993;34:137–42.
-
McCord ME, Bacharach SL, Bonow RO, Dilsizian V, Cuocolo A, Freedman N. Misalignment between PET transmission and emission scans: its effect on myocardial imaging. Journal of Nuclear Medicine. 1992;33:1209–1214.
-
Loghin C, Sdringola S, Gould KL. Common artifacts in PET myocardial perfusion images due to attenuation-emission misregistration: clinical significance, causes, and solutions. Journal of Nuclear Medicine. 2004;45:1029–39.
1.4.2 Cardiac Motion Artefacts
Case 5
History
-
63-year-old male with no risk factors
-
Non-anginal chest pain
PET/CT Images (Figs. 1.10 and 1.11)
MPI shows a mild reversible perfusion defect of the apical region. The quantitative values (see Table 1.1; non-motion corrected data (NMC data) show an abnormal (too high) MBF at rest in the RCA territory resulting in an impairment of the MFR in the same territory
A quality check for LV motion during acquisition shows a downshift of the LV during the LV cavity filling at rest and an upshift of myocardial wall in the stress study (Fig. 1.11).
After correction for patient movements, both MBF and MFR values normalize (Table 1.1).
Findings
-
In the originally reconstructed images, MPI shows mild reversible perfusion defect in the infero-apical segments.
-
In the study non-corrected for motion, MFR appears severely impaired in the RCA territory (Regadenoson-stress test) due to abnormally elevated rest MBF.
-
Study reconstructed after motion correction shows values within normal limits.
Correlative Imaging
-
Coronary angiography showing normal coronary anatomy (Fig. 1.12)
Differential Diagnosis
-
Obstructive CAD with single vessel myocardial ischaemia
Teaching Points
-
A quality control of possible patient motion during acquisition is mandatory as it can affect results.
-
After Regadenoson injection, an increase in breathing excursion and, consequently, a cardiac upward shift is commonly observed.
Management
-
Reassurance and risk factor management
Further Reading
-
Vleeming E, Lazarenko S, van der Zant F, Pan X, Declerck J, Wondergem M, et al. Cardiac Displacement During13N-Ammonia Myocardial Perfusion PET/CT: Comparison Between Adenosine- and Regadenoson-Induced Stress. Journal of Nuclear Medicine Technology. 2017;46:114–122.
-
Schleyer P, O’Doherty M, Barrington S, Morton G, Marsden P. Comparing approaches to correct for respiratory motion in NH3 PET-CT cardiac perfusion imaging. Nuclear Medicine Communications. 2013;34:1174–1184.
Case 6
History
-
A 62-year-old female with obesity and HIV infection was referred for a rest/stress myocardial perfusion PET/CT to evaluate for atypical angina and dyspnoea (Figs. 1.13 and 1.14).
PET/CT Images
Summed rest and Regadenoson-stress myocardial perfusion PET/CT images obtained with 13N-ammonia. The display is as in case # 1. The images demonstrate blurring of the mid and basal anterolateral and inferolateral walls, resulting in a perfusion defect of moderate intensity in these LV segments (arrows), showing complete reversibility
Findings
-
The summed stress images demonstrate blurring of the lateral wall with an apparent perfusion defect (arrows).
-
However, the end-diastolic images demonstrate normal regional myocardial perfusion.
Differential Diagnosis
-
Obstructive CAD
Correlative Imaging
-
None
Management
-
Reassurance and risk factor management
Teaching Points
-
Blurring of the lateral wall due to cardiac motion is common and can lead to false positive interpretation.
-
Inspection of the relatively motion-free end-diastolic images helps differentiate a real perfusion defect from one resulting from excessive cardiac motion.
Further Reading
-
Di Carli M, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore S. Clinical Myocardial Perfusion PET/CT. Journal of Nuclear Medicine. 2007;48:783–793.
1.4.3 Ammonia Lateral Defect
Case 7
History
-
40 years old female.
-
Asymptomatic. No cardiovascular risk factors. Pre-surgical (kidney donor).
PET/CT Images (Fig. 1.15)
Findings
-
Decreased perfusion in the lateral wall of the left ventricle at rest and stress.
-
Normal thickening, normal LV function with a rest LVEF of 79% up to 87% at peak stress; MBF and MFR.
Differential Diagnosis
-
Ischaemic disease in the left circumflex territory
Correlative Image
-
Coronary Computed Tomography (Fig. 1.16)
Management
-
None
Teaching Points
-
Decreased counts in the lateral wall of the left ventricle with 13N-Ammonia PET/CT occur frequently. Misalignment of the PET and CT images should always be considered and checked.
-
The challenge is to differentiate between a real perfusion defect vs artefact.
Further Reading
-
Klingensmith W, Noonan C, Goldberg J, Buchwald D, Kimball J, Manson S. Decreased Perfusion in the Lateral Wall of the Left Ventricle in PET/CT Studies with 13N-Ammonia: Evaluation in Healthy Adults. Journal of Nuclear Medicine Technology. 2009;37:215–219.
-
Nakazato R, Berman D, Alexanderson E, Slomka P. Myocardial perfusion imaging with PET. Imaging in Medicine. 2013;5:35–46.
1.4.4 Non-Responder to Coronary Vasodilators
Case 8
History
-
67-year-old male with type 2 diabetes
-
Risk factors: dyslipidaemia, hypertension
-
At the age of 35, multivessel revascularization by CABG
-
At the age of 57, PCI on multiple vessels
-
13NH3 PET/CT requested for evaluation of residual ischaemia
PET/CT Images
-
A first study showed normal perfusion both at stress and rest, but low MFR values (Fig. 1.17).
Enquired after completion of the imaging procedure the patient admitted that he had a cup of coffee 30 min before the stress test, despite detailed instructions given at registration. The study was repeated with repeated instructions to the patient to avoid caffeine (Fig. 1.18).
Findings
-
No reversible perfusion defects on initial MPI
-
No flow reserve after dipyridamole infusion
-
Test deemed falsely negative due to caffeine intake
Management
-
Repetition of the study after instructions to the patient to avoid caffeine
Teaching Points
-
Even low levels of serum caffeine may considerably worsen global and regional perfusion heterogeneity, leading to false positive and false negative perfusion results, and potentially impairing optimal patient risk stratification and management.
-
Current guidelines recommend avoidance of adenosine, dipyridamole and regadenoson if caffeine has been consumed within 12 h.
-
Patients should be strictly reminded to avoid caffeine and xanthine (coffee, tea, etc.) when pharmacologic stress testing is planned.
-
Personnel should enquire at reception if instructions have been complied with.
Further Reading
-
Kitkungvan D, Bui L, Johnson N, Patel M, Roby A, Vejpongsa P, et al. Quantitative myocardial perfusion positron emission tomography and caffeine revisited with new insights on major adverse cardiovascular events and coronary flow capacity. European Heart Journal - Cardiovascular Imaging. 2019;20:751–762.
-
Dorbala S, Ananthasubramaniam K, Armstrong I, Chareonthaitawee P, DePuey E, Einstein A, et al. Single Photon Emission Computed Tomography (SPECT) Myocardial Perfusion Imaging Guidelines: Instrumentation, Acquisition, Processing, and Interpretation. Journal of Nuclear Cardiology. 2018;25:1784–1846.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
The opinions expressed in this chapter are those of the author(s) and do not necessarily reflect the views of the [NameOfOrganization], its Board of Directors, or the countries they represent
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 3.0 IGO license (http://creativecommons.org/licenses/by/3.0/igo/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the [NameOfOrganization], provide a link to the Creative Commons license and indicate if changes were made.
Any dispute related to the use of the works of the [NameOfOrganization] that cannot be settled amicably shall be submitted to arbitration pursuant to the UNCITRAL rules. The use of the [NameOfOrganization]'s name for any purpose other than for attribution, and the use of the [NameOfOrganization]'s logo, shall be subject to a separate written license agreement between the [NameOfOrganization] and the user and is not authorized as part of this CC-IGO license. Note that the link provided above includes additional terms and conditions of the license.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Di Carli, M.F. et al. (2022). Technical Considerations for Cardiac PET/CT. In: Di Carli, M.F., Dondi, M., Giubbini, R., Paez, D. (eds) IAEA Atlas of Cardiac PET/CT. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-64499-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-662-64499-7_1
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-64498-0
Online ISBN: 978-3-662-64499-7
eBook Packages: MedicineMedicine (R0)