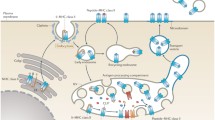
Abstract
Class I molecules of the major histocompatibility complex (MHC) present peptides derived from endogenous proteins at the cell surface. During viral infection or malignant transformation a different set of peptides is displayed by MHC class I molecules. These antigen-loaded class I complexes are recognized by cytotoxic T cells via the T cell receptors as nonself, thus leading to the destruction of the abnormal cell.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Bjorkman PJ, Strominger JL, Wiley DC. Crystallization and X-ray diffraction studies on the histocompatibility antigens HLA-A2 and HLAA28 from human cell membranes. J Mol Biol 1985; 182: 205–210.
Bjorkman PJ, Saper MA, Samraoui B et al. Structure of the human class I histocompatibility antigen HLA-A2. Nature 1987; 329: 506–512.
Garrett TPJ, Saper MA, Bjorkman PJ et al. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature 1989; 342: 692–696.
Guo HC, Jardetzky TS, Garrett TPJ et al. Different length peptides bind to HLA-Aw68 similarly at their ends but bulge out in the middle. Nature 1992; 360: 364–366.
Collins EJ, Garboczi DN, Wiley DC. 3-dimensional structure of a peptide extending from one end of a class-I MHC binding-site. Nature 1994; 371: 626–629.
Glynne R, Powis SH, Beck S et al. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature 1991; 353: 357–360.
Martinez CK, Monaco JJ. Homology of proteasome subunits to a major histocompatibility complex-linked imp gene. Nature 1991; 353: 664–667.
Kelly A, Powis SH, Glynne R et al. Second proteasome-related gene in the human MHC class II region. Nature 1991; 353: 667–668.
Belich MP, Glynne RJ, Senger G et al. Proteasome components with reciprocal expression to that of the MHC-encoded Lmp proteins. Current Biol 1994; 4: 769–776.
Früh K, Gossen M, Wang KN et al. Displacement of housekeeping proteasome subunits by MHC-encoded Lmps-a newly discovered mechanism for modulating the multicatalytic proteinase complex. EMBO J 1994; 13: 3236–3244.
Akiyama KY, Yokota KY, Kagawa S et al. cDNA cloning and interferon down-regulation of proteasomal subunit X and subunit Y. Science 1994; 276: 1231–1234.
Driscoll J, Brown MG, Finley D et al. MHC-linked Lmp gene products specifically alter peptidase activities of the proteasome. Nature 1993; 365: 262–264.
Gaczynska M, Rock KL, Goldberg AL. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature 1993; 365: 264–267.
Ehring B, Meyer TH, Eckerskorn C et al. Effects of major-histocompatibility-complex-encoded subunits on the peptidase and proteolytic activities of human 20S proteasomes-cleavage of proteins and antigenic peptides. Eur J Biochem 1996; 235: 404–415.
van Kaer L, Ashton-Rickardt PG, Eichelberger M et al. Altered peptidase and viral-specific T cell response in lmp2 mutant mice. Immunity 1994; 1: 533–541.
Fehling HJ, Swat W, Laplace C et al. MHC class I expression in mice lacking the proteasome subunit Lmp7. Science 1994; 265: 1234–1237.
Rock KL, Gramm C, Rothstein L et al. Inhibitors of the proteasome block the degradation of most cell-proteins and the generation of peptides presented on MHC class-I molecules. Cell 1994; 78: 761–771.
Michalek MT, Grant EP, Gramm C et al. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature 1993; 363: 552–554.
Townsend A, Öhlen C, Bastin J et al. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature 1989; 340: 443–448.
Cerundolo V, Alexander J, Anderson K et al. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature 1990; 345: 449–452.
Hosken NA, Bevan MJ. Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science 1990; 248: 367–369.
Trowsdale J, Hanson I, Mockridge I et al. Sequences encoded in the class II region of the MHC related to the `ABC’ superfamily of transporters. Nature 1990; 348: 741–744.
Spies T, Bresnahan M, Bahram S et al. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature 1990; 348: 744–747.
Deverson EV, Gow IR, Coadwell WJ et al. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature 1990; 348: 738–741.
Monaco JJ, Cho S, Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science 1990; 250: 1723–1726.
Spies T, DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature 1991; 351: 323–324.
Powis SJ, Townsend ARM, Deverson EV et al. Restoration of antigen presentation to the mutant cell line RMA-S by an MHC-linked transporter. Nature 1991; 354: 528–531.
Attaya M, Jameson S, Martinez CK et al. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature 1992; 355: 647–649.
Kelly AP, Powis SH, Kerr L-A et al. Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature 1992; 355: 641–644.
Kleijmeer M, Kelly A, Geuze HJ et al. Location of MHC-encoded transporters in the endoplasmic reticulum and cis-Golgi. Nature 1992; 357: 342–344.
Lévy F, Gabathuler R, Larsson R et al. ATP is required for in vitro assembly of MHC class I antigens but not for transfer of peptides across the ER membrane. Cell 1991; 67: 265–274.
Koppelman B, Zimmerman D, Walter P et al. Evidence for peptide transport across microsomal membranes. Proc Natl Acad Sci USA 1992; 89: 3908–3912.
Neefjes JJ, Momburg F, Hämmerling GJ. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science 1993; 261: 769–771.
Androlewicz MJ, Anderson KS, Cresswell P. Evidence that transporter associated with antigen processing translocate a major histocompatibility complex cass I-binding peptide into the endoplasmic reticulum in an ATP-dependent manner. Proc Natl Acad Sci USA 1993; 90: 9130–9134.
Shepherd JC, Schumacher TN, P.G. A-R et al. tapl-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell 1993; 74: 577–84.
Meyer TH, van Endert PM, Uebel S et al. Functional expression and purification of the ABC transporter complex-associated with antigen-processing (TAP) in insect cells. FEBS Lett 1994; 351: 443–447.
Powis SH, Mockridge I, Kelly A et al. Polymorphism in a second ABC transporter gene located within the class II region of the human major histocompatibility complex. Proc Natl Acad Sci USA 1992; 89: 1463–1467.
Colonna M, Bresnahan M, Bahram S et al. Allelic variants of the human putative peptide transporter involved in antigen processing. Proc Natl Acad Sci USA 1992; 89: 3932–3936.
Carrington M, Colonna M, Spies T et al. Haplotypic variation of the transporter associated with antigen processing (TAP) genes and their extension of HLA class II region haplotypes. Immunogenetics 1993; 37: 266–273.
Powis SJ, Deverson EV, Coadwell WJ et al. Effect of polymorphism of an MHC-linked transporter on the peptides assembled in a class I molecule. Nature 1992; 357: 211–215.
Heemels MT, Schumacher TNM, Wonigeit K et al. Peptide translocation by variants of the transporter associated with antigen processing. Science 1993; 262: 2059–2063.
Wang P, Gyllner G, Kvist S. Selection and binding of peptides to human transporters associated with antigen processing and rat cim-a and -b. J Immunol 1996; 157: 213–220.
Higgins CF. ABC transporters: from microorganisms to man. Ann Rev Cell Biol 1992; 8: 67–113.
Walker JE, Saraste M, Runswick MJ et al. Distantly related sequences in the a-and 13-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1982; 1: 945–951.
Dassa E, Hofnung M. Sequence of gene malG in E. coli K12: homologies between integral membrane components from binding protein-dependent transport systems. EMBO J 1985; 4: 2287–2293.
Dassa E. Cellular localization of the MalG protein from the maltose transport system in Escherichia coli K12. Mol Gen Genetics 1990; 222: 33–36.
Cotten JF, Ostedgaard LS, Carson MR et al. Effect of cystic fibrosis-associated mutations in the 4th intracellular loop of cystic-fibrosis trans-membrane conductance regulator. J Biol Chem 1996; 271: 21279–21284.
Hughes AL. Evolution of the ATP-binding-cassette transmembrane transporters of vertebrates. Mol Biol & Evol 1994; 11: 899–910.
Michaelis S, Berkower C. Sequence comparison of yeast ATP-binding cassette proteins. In: Cold Spring Harbor Symposia on Quantitative Bi-ology, Volume LX, Cold Spring Harbor Laboratory Press, 1995.
Dean M, Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Gen Dev 1995; 5: 779–785.
Chen CJ, Clark D, Ueda K et al. Genomic organization of the human multidrug resistance (MDR1) gene and origin of P-glycoproteins. J Biol Chem 1990; 265: 506–514.
Bult CJ, White O, Zhou L et al. Complete genome sequence of the methanogenic archaeon Methanococcus jannaschii. Science 1996; 273: 1058–1072.
Raymond M, Gros P, Whiteway M et al. Functional complementation of yeast Ste6 by a mammalian multidrug resistance mdr gene. Science 1992; 256: 232–234.
Volkman SK, Cowman AF, Wirth DF. Functional complementation of the ste6 gene of Saccharomyces cerevisiae with the pfmdrl gene of Plasmodium falciparum. Proc Natl Acad Sci USA 1995; 92: 8921–8925.
Ruetz S, Brault M, Kast C et al. Functional expression of the multidrug resistance-associated protein mrp in the yeast Saccharomyces cerevisiae. J Biol Chem 1996; 271: 4154–4160.
Müller KM, Ebensperger C, Tampé R. Nucleotide binding to the hydrophilic C-terminal domain of the transporter associated with antigen processing (TAP). J Biol Chem 1994; 269: 14032–14037.
Wang K, Früh K, Peterson PA et al. Nucleotide binding of the C-terminal domains of the major histocompatibility complex-encoded transporter expressed in Drosophila melanogaster cells. FEBS Lett 1994; 350: 337–341.
Russ G, Esquivel F, Yewdell JW et al. Assembly, intracellular localization, and nucleotide-binding properties of the human peptide transporters tapi and tap2 expressed by recombinant vaccinia viruses. J Biol Chem 1995; 270: 21312–21318.
Meyer TH. ed. Functional expression and characterization of the MHCencoded peptide transporter (TAP). Technical University Munich. 1996: Ph.D. Thesis.
van Endert PM, Tampé R, Meyer TH et al. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity 1994; 1: 491–500.
Uebel S, Meyer TH, Kraas W et al. Requirements for peptide binding to the human transporter associated with antigen-processing revealed by peptide scans and complex peptide libraries. J Biol Chem 1995; 270: 18512–18516.
Chen HL, Gabrilovic D, Tampé R et al. A functionally defective allele of tap/ results in loss of MHC class I antigen presentation in a human lung cancer. Nature Gen 1996; 13: 210–213.
Holland I, Blight M. Structure and function of HlyB, the ABC transporter essential for haemolysin secretion from Escherichia coli. Biochim Biophis Acta (submitted for publication) 1996;
Dassa E, Muir S. Membrane topology of Ma1G, an inner membrane protein from the maltose transport system of Escherichia coli. Mol Microbiol 1993; 7: 29–38.
Ehrle R, Pick C, Ulrich R et al. Characterization of transmembrane domains 6, 7, and 8 of Ma1F by mutational analysis. J Bacteriol 1996; 178: 2255–2262.
Chang XB, Hou YX, Jensen TJ et al. Mapping of cystic-fibrosis trans-membrane conductance regulator membrane topology by glycosylation site insertion. J Biol Chem 1994; 269: 18572–18575.
Geller D, Taglicht D, Edgar R et al. Comparative topology studies in Saccharomyces cerevisiae and in Escherichia coli—the N-terminal half of the yeast ABC protein Ste6. J Biol Chem 1996; 271: 13746–13753.
Kast C, Canfield V, Levenson R et al. Membrane topology of P-glycoprotein as determined by epitope insertion-transmembrane organization of the N-terminal domain of MDR3. Biochemistry 1995; 34: 4402–4411.
Kast C, Canfield V, Levenson R et al. Transmembrane organization of mouse P-glycoprotein determined by epitope insertion and immunofluorescence. J Biol Chem 1996; 271: 9240–9248.
Zhang J-T, Ling V. Study of membrane orientation and glycosylated extracellular loops of mouse P-glycoprotein by in vitro translation. J Biol Chem 1991; 266: 18224–18232.
Zhang J-T, Duthie M, Ling V. Membrane topology of the N-terminal half of the hamster P-glycoprotein molecule. J Biol Chem 1993; 268: 15101–15110.
Loo TW, Clarke DM. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem 1995; 270: 843–848.
Loo TW, Clarke DM. Mutational analysis of the predicted first trans-membrane segment of each homologous half of human P-glycoprotein suggests that they are symmetrically arranged in the membrane. J Biol Chem 1996; 271: 15414–15419.
van Iwaarden P, Pastore J, Konings W et al. Construction of a functional lactose permease devoid of cysteine residues. Biochemistry 1991; 30: 9595–9600.
Frillingos S, Kaback H. Cysteine-scanning mutagenesis of helix VI and the flanking hydrophilic domains in the lactose permease of Escherichia coli. Biochemistry 1996; 35: 5333–5338.
Powis SJ, Young LL, Joly E et al. The rat cim effect-tap allele-dependent changes in a class-I MHC anchor motif and evidence against C-terminal trimming of peptides in the ER. Immunity 1996; 4: 159–165.
Momburg F, Armandola EA, Post M et al. Residues in tap2 peptide transporters controlling substrate-specificity. J Immunol 1996; 156: 1756–1763.
Androlewicz MJ, Cresswell P. Human transporters associated with antigen processing possess a promiscuous peptide binding site. Immunity 1994; 1: 7–14.
Nijenhuis M, Schmitt S, Armandola EA et al. Identification of a contact region for peptide on the tapi chain of the transporter associated with antigen processing. J Immunol 1996; 156: 2186–2195.
Armandola EA, Momburg F, Nijenhuis M et al. A point mutation in the human transporter associated with antigen-processing (tap2) alters the peptide-transport specificity. Eur J Immun 1996; 26: 1748–1755.
Greenberger LM. Major photoaffinity drug labeling sites for iodoaryl azidoprazosin in P-glycoprotein are within, or immediately C-terminal to, transmembrane domain 6 and domain 12. J Biol Chem 1993; 268: 11417–11425.
Morris DI, Greenberger LM, Bruggemann EP et al. Localization of the forskolin labeling sites to both halves of P-glycoprotein-similarity of the sites labeled by forskolin and prazosin. Mol Pharmacol 1994; 46: 329–337.
Koopmann JO, Post M, Neefjes JJ et al. Translocation of long peptides by transporters associated with antigen-processing (TAP). Eur J Immun 1996; 26: 1720–1728.
Momburg F, Roelse J, Hämmerling GJ et al. Peptide size selection by the major histocompatibility complex-encoded peptide transporter. J Exp Med 1994; 179: 1613–1623.
Heemels MT, Ploegh HL. Substrate-specificity of allelic variants of the TAP peptide transporter. Immunity 1994; 1: 775–784.
Momburg F, Roelse J, Howard JC et al. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature 1994; 367: 648–651.
Androlewicz MJ, Cresswell P. How selective is the transporter associated with antigen-processing. Immunity 1996; 5: 1–5.
Neisig A, Roelse J, Sijts AJA et al. Major differences in transporter associated with antigen presentation (TAP)-dependent translocation of MHC class I-presentable peptides and the effect of flanking sequences. J Immunol 1995; 154: 1273–1279.
Schumacher TN, Kantesaria DV, Heemels MT et al. Peptide length and sequence specificity of the mouse taps/tap2 translocator. J Exp Med 1994; 179: 533–540.
Androlewicz MJ, Ortmann B, van Endert PM et al. Characteristics of peptide and major histocompatibility complex class-I p(2)-microglobulin binding to the transporters associated with antigen-processing (tapi and tap2). Proc Natl Acad Sci USA 1994; 91: 12716–12720.
Ortmann B, Androlewicz MI, Cresswell P. MHC class I/13 2-microglobulin complexes associate with TAP transporters before peptide binding. Nature 1994; 368: 864–867.
Suh WK, Cohen-Doyle MF, Früh K et al. Interaction of MHC class I molecules with the transporter associated with antigen processing. Science 1994; 264: 1322–1326.
Peace-Brewer AL, Tussey LG, Matsui M et al. A point mutation in HLAA*0201 results in failure to bind the TAP complex and to present virus-derived peptides to CTL. Immunity 1996; 4: 505–514.
Grandea AG, Androlewicz MJ, Athwal RS et al. Dependence of peptide binding by MHC class-I molecules on their interaction with TAP. Science 1995; 270: 105–108.
Anderson K, Cresswell P, Gammon M et al. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J Exp Med 1991; 174: 489–492.
Sadasivan B, Lehner PJ, Ortmann B et al. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class-I molecules with TAP. Immunity 1996; 5: 103–114.
Caillat ZS, Bertin E, Timsit J et al. Protection from insulin-dependent diabetes mellitus is linked to a peptide transporter gene. Eur J Immun 1993; 23: 1784–1788.98. Wordsworth BP, Pile KD, Gibson K et al. Analysis of the MHC-encoded transporters tapi and tap2 in rheumatoid arthritis: linkage with DR4 accounts for the association with a minor tap2 allele. Tiss Antig 1993; 42: 153–5.
Moinsteisserenc H, Semana G, Alizadeh M et al. Tap2 gene polymorphism contributes to genetic susceptibility to multiple-sclerosis. Human Immunol 1995; 42: 195–202.
Vandevyver C, Stinissen P, Cassiman JJ et al. Tap-1 and Tap-2 transporter gene polymorphisms in multiple-sclerosis-no evidence for disease association with tap. J Neuroimmunol 1994; 54: 35–40.
Vandevyver C, Geusens P, Cassiman JJ et al. Peptide transporter genes (tap) polymorphisms and genetic susceptibility to rheumatoid-arthritis. British J Rheumatol 1995; 34: 207–214.
Obst R, Armandola EA, Nijenhuis M et al. TAP polymorphism does not influence transport of peptide variants in mice and humans. Eur J Immun 1995; 25: 2170–2176.
de la Salle H, Hanau D, Fricker D et al. Homozygous human TAP peptide transporter mutation in HLA class-I deficiency. Science 1994; 265: 237–241.
Cromme FV, Airey J, Heemels MT et al. Loss of transporter protein, encoded by the tapi gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med 1994; 177: 505–509.
Kaklamanis L, Townsend A, Doussisanagnostopoulou IA et al. Loss of major histocompatibility complex-encoded transporter associated with antigen presentation (tap) in colorectal-cancer. American J Pathol 1994; 145: 505–509.
Rotemyehudar R, Winograd S, Sela S et al. Down-regulation of peptide transporter genes in cell-lines transformed with the highly oncogenic adenovirus-12. J Exp Med 1994; 180: 477–488.
Seliger B, Höhne A, Knuth A et al. Analysis of the major histocompatibility complex class I antigen presentation machinery in normal and malignant renal cells: evidence for deficiencies associated with transformation and progression. Cancer Res 1996; 56: 1756–1760.
Seliger B, Höhne A, Knuth A et al. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and Lmp expression. Clin Cancer Res 1996; 2: 1427–1433.
York IA, Roop C, Andrews DW et al. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 1994; 77: 525–535.
Früh K, Ahn K, Djaballah H et al. A viral inhibitor of peptide transporters for antigen presentation. Nature 1995; 375: 415–418.
Hill A, Jugovic P, York I et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature 1995; 375: 411–415.
Ahn K, Meyer TH, Uebel S et al. Molecular mechanism and species-specificity of TAP inhibition by herpes-simplex virus protein ICP47. EMBO J 1996; 15: 3247–3255.
Tomazin R, Hill AB, Jugovic P et al. Stable binding of the Herpes Simplex virus ICP47 protein to the peptide binding-site of TAP. EMBO J 1996; 15: 3256–3266.
Beck S, Kelly A, Radley E et al. DNA sequence analysis of 66 kb of the human MHC class II region encoding a cluster of genes for antigen processing. J Mol Biol 1992; 228: 433–441.
Chen CJ, Chin JE, Ueda K et al. Internal duplication and homology with bacterial transport proteins in the MDR1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 1986; 47: 381–389.
van der Bliek AM, Kooiman PM, Schneider C et al. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene 1988; 71: 401–411.
Riordan JR, Rommens JM, Kerem B et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066–73.
Mosser J, Douar AM, Sarde CO et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993; 361: 726–730.
Kamijo K, Kamijo T, Ueno I et al. Nucleotide sequence of the human 70 kDa peroxisomal membrane protein: a member of ATP-binding cassette transporters. Biochim Biophys Acta 1992; 1129: 323–327.
Thomas PM, Cote GJ, Wohllk N et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 1995; 268: 426–429.
McGrath JP, Varshaysky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature 1989; 340: 400–404.
Kuchler K, Sterne RE, Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J 1989; 8: 3973–3984.
Dean M, Allikmets R, Gerrard B et al. Mapping and sequencing of two yeast genes belonging to the ATP-binding cassette superfamily. Yeast 1994; 10: 377–383.
Leighton J, Schatz G. An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J 1995; 14: 188–195.
Ortiz DF, Kreppel L, Speiser DM et al. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J 1992; 11: 3491–3491.
Hess J, Wels W, Vogel M et al. Nucleotide sequence of a plasmid-encoded haemolysin determinant and its comparison with a corresponding chromosomal haemolysin sequence. FEMS Microbiol Lett 1986; 34: 1–11.
Devereaux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 1984; 12: 387.
Felsenstein J. PHYLIP: phylogeny inference package (version 3.56). Cladistics 1989; 5: 164.
Claros MG, von Heijne G. Prediction of transmembrane segments in integral membrane proteins, and putative topologies, using several algorithms. CABIOS 1994; 10: 685–686.
Gottesmann MM, Hrycyna CA, Schoenlein PV et al. Genetic analysis of the multidrug transporter. Ann Rev Genet 1995; 29: 607–649.
Cheng SH, Gregory RJ, Marshall J et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990; 63: 827–834.
Rights and permissions
Copyright information
© 1997 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tampé, R., Urlinger, S., Pawlitschko, K., Uebel, S. (1997). The Transporters Associated with Antigen Processing (TAP). In: Unusual Secretory Pathways: From Bacteria to Man. Molecular Biology Intelligence Unit. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-22581-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-662-22581-3_4
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-22583-7
Online ISBN: 978-3-662-22581-3
eBook Packages: Springer Book Archive