Abstract
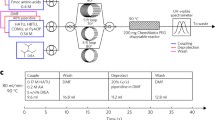
Peptide synthesis is a repetitive procedure schematically shown in Fig. 1. Each cycle of deprotection, wash, coupling and wash introduces one amino acid building block (residue) to the growing chains anchored covalently to an insoluble solid support via the carboxy terminus. Temporary protection for the α-amino group of the incoming residue, which is removed in each cycle (deprotection), ensures that the residue is coupled to the growing chain only once. Coupling requires prior activation of the carboxy group of the incoming residue, as explained in more detail below. To prevent undesired reactions, the side-chain functional groups are also protected during the synthesis and are deprotected together at the end of the synthesis. Between individual operations, the support has to be thoroughly washed in order to remove excess reagents from the growing chains. This is actually the most important feature of solid-phase peptide synthesis (SPPS) for which Bruce Merrifield was awarded the Nobel Prize in 1984. Typically, the peptides are cleaved from the support together with the side-chain protecting groups, but there are several experimental procedures, which require solid-phase-bound peptides. The SPOT technique uses membranes as the solid support and enables the parallel synthesis and testing of hundreds to thousands of peptides at different locations on one membrane (Frank 1992).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Bernatowicz MS, Daniels, SB, Köster H (1989) A comparison of acid labile linkage agents for the synthesis of peptide C-terminal amides. Tetrahedron Lett 30: 4645–4648
Bray AM, Maeji NJ, Geysen HM (1990) The simultaneous multiple production of solution phase peptides; assessment of the Geysen method of simultaneous peptide synthesis. Tetrahedron Lett 31: 5811–5814
Chan WC, White PD (2000) Fmoc solid phase peptide synthesis: a practical approach. Oxford University Press, Oxford
Fields GB, Noble RL (1990) Solid phase peptide synthesis utilising 9fluorenylmethoxycarbonyl amino acids. Int J Peptide Protein Res 35: 161–214
Frank R (1992) Spot synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 48: 9217–9232
Frank R, Overwin H (1996) Spot synthesis. In: Morris GE (ed) Epitope mapping protocols. Methods in molecular biology. Humana, Totowa, New Jersey, pp 149–169
Holmes CP, Jones DG (1995) Reagents for combinatorial organic synthesis: development of a new o-nitrobenzyl photolabile linker for solid-phase synthesis. J Org Chem 60: 2318–2319
Pilawa S, Zander N, Frank R (2001) Optimized reaction conditions for the direct esterification of protected amino acids to cellulose membrane supports by the SPOT technique. In: Epton R (ed) Innovation and perspectives in solid phase synthesis and combinatorial libraries. Proc 6th Int Symp, York, 1999. Mayflower Worldwide, Birmingham, UK, pp 337–338
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2002 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Zander, N., Gausepohl, H. (2002). Chemistry of Fmoc Peptide Synthesis on Membranes. In: Koch, J., Mahler, M. (eds) Peptide Arrays on Membrane Supports. Springer Lab Manuals. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-09229-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-662-09229-3_2
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-07639-8
Online ISBN: 978-3-662-09229-3
eBook Packages: Springer Book Archive