Abstract
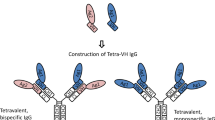
Diabodies are small dimeric bivalent or bispecific antibody fragments formed by cross-over pairing of two single-chain VH-VL fragments (Holliger et al., 1993, Whitlow et al., 1994). Dimer formation is favoured by reducing the linker length between the VH-VL domains from 15–20 amino acids, normally used to generate scFv fragments, to approximately 5 amino acids (Fig. lA). Further reduction of the linker can result in the formation of trimeric or even tetrameric molecules (triabodies, tetrabodies) (Kortt et al., 1997; Le Gall et al., 1999). As shown by crystallographic studies, the two binding sites of a diabody molecule are facing away from each other (Perisic et al., 1994) (Fig. 1B). Bivalent diabodies are generated by dimeric assembly of two identical VH-VL chains (homodimers). Due to the presence of two antigen binding sites, bivalent diabodies exhibit an increased functional affinity (Fitzgerald et al., 1997). The expression of two fragments of the format VHA-VLB and VHB-VLA in the same cell results in formation of heterodimers recognising two different antigens, but may also lead to the formation of non-functional homodimers (Fig. 1 C).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Alt M, Müller R, Kontermann RE (1999) Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin γ1 Fc or CH3 region. FEBS Lett 454;90–94
Alfthan K, Takkinen K, Sizmann D, Söderlund H, Teeri TT (1995) Properties of a single-chain antibody containing different linker peptides. Protein Eng 8;725–731
Brüsselbach S, Korn T, Völkel T, Müller R, Kontermann RE (1999) Enzyme recruitment and tumor cell killing in vitro by a secreted bispecific single-chain diabody. Tumor Targeting 4;115–123
Desplancq D, King DJ, Lawson ADG, Mountain A (1994) Multimerization behaviour of single chain Fv variants for the tumour-binding antibody B72.3. Protein Eng 8;1027–1033
Fitzgerald K, Holliger P, Winter G (1997) Improved tumour targeting by disulphide stabilized diabodies expressed in Pichia pastoris. Protein Eng 10;1221–1225
Helfrich W, Kroesen BJ, Roovers RC, Westers L, Molema G, Hoogenboom HR, de Leij L (1998) Construction and characterization of a bispecific diabody for retargeting T cells to human carcinoma. Int J Cancer 76,232–239
Holliger P, Brissinck J, Williams RL, Thielemans K, Winter G (1996) Specific killing of lymphoma cells by cytotoxic T-cells mediated by a bispecific diabody. Protein Eng 9;299–305
Holliger P, Prospero T, Winter G (1993) “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA 90;6444–6448
Holliger P, Wing M, Pound JD, Bohlen H, Winter G (1997) Retargeting serum immunoglobulin with bispecific diabodies. Nat Biotechn 15;632–636
Kipriyanov SM, Moldenhauer G, Strauss G, Little M (1998) Bispecific CD3xCD19 diabody for T cell-mediated lysis of malignant human B cells. Int T Cancer 77;763–772
Kipriyanov SM, Moldenhauer G, Strauss G, Little M, Kontermann RE, Martineau P, Cummings CE, Karpas A, Allen D, Derbyshire E, Winter G (1997a) Enzyme immunoassays using bispecific diabodies. Immunotechnology 3:137–144
Kontermann RE, Müller R (1999) Intracellular and cell surface displayed single-chain diabodies. J Immunol Meth 226;179–188
Kontermann RE, Wing MG, Winter G (1997b) Complement recruitment using bispecific diabodies. Nat Biotechnol 15;629–631
Le Gall F, Kipriyanov SM, Moldenhauer G, Little M (1999) Di, tri and tetrameric single chain Fv antibody fragments against human CD19: effect of valency on cell binding. FEBS Lett 453;164–168
Low NM, Holliger P, Winter G (1996) Mimicking somatic hypermutation: Affinity maturation of antibodies displayed on bacteriophage using bacterial mutator strain. J Mol Biol 260;359–368
Krebs B, Griffin H, Winter G, Rose-John S (1998) Recombinant human single-chain Fv antibodies recoginizing human interleukin-6: specific targeting of cytokine-secreting cells. T Biol Chem 273:2858–2865
Kortt A, Lah M, Oddie GW, Gruen CL, Burns JE, Pearce LA, Atwell JL, McCoy AK, Howlett GJ, Metzger DW, Webster RG, Hudson PJ (1997) Single-chain Fv fragments of anti-neuraminidase antibody NC 10 containing five- and ten-residue linkers form dimers and with zero-residue linker a trimer. Protein Eng 10: 423–433
Kortt A, Lah M, Oddie GW, Gruen CL, Burns JE, Pearce LA, Atwell JL, McCoy AK, Howlett GJ, Metzger DW, Webster RG, Hudson PJ; Perisic O, Webb PA, Holliger P, Winter G, Williams RL (1994) Crystal structure of a diabody, a bivalent antibody fragment. Structure 2;1217–1226
Power BE, Ivancic N, Harley VR, Webster RG, Kortt AA, Irving RA, Hudson PJ (1992) High-level temperature-induced synthesis of an antibody VH domain in Escherichia coli using the PelB secretion signal. Gene 113;95–99
Whitlow M, Filpula D, Rollence ML, Feng SL, Wood JF (1994) Multivalent Fvs: characterization of single-chain Fv oligomers and preparation of a bispecific Fv. Protein Eng 7;1017–1026
Zhu Z, Presta LG, Zapata G, Carter P (1997) Remodeling domain interface to enhance heterodimer formation. Protein Sci 6;781–788
Zhu Z, Zapata G, Shalaby R, Snedecor B, Chen H, Carter P (1996) High level secretion of a humanized bispecific diabody from Escherichia coli. Bio/Technol 14;192–196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2001 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Korn, T., Völkel, T., Kontermann, R.E. (2001). Bivalent and Bispecific Diabodies and Single-chain Diabodies. In: Kontermann, R., Dübel, S. (eds) Antibody Engineering. Springer Lab Manuals. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-04605-0_42
Download citation
DOI: https://doi.org/10.1007/978-3-662-04605-0_42
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-41354-7
Online ISBN: 978-3-662-04605-0
eBook Packages: Springer Book Archive