Abstract
This chapter highlights the role of imaging, particularly magnetic resonance imaging (MRI) and fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT), as a “compass” along the therapeutic path in patients with malignant diseases of the uterus.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
To describe the role of MRI and PET/CT for initial treatment selection, planning, and response assessment in uterine malignancies
-
To explain eligibility criteria for fertility preservation in cervical cancer
-
To highlight combined MR features that may distinguish uterine sarcoma from atypical leiomyoma (LM)
18.1 Cervical Cancer
18.1.1 Epidemiology and Diagnosis
Cervical cancer is the fourth most common malignancy among women worldwide [1, 2]. Most cases and deaths occur in developing countries, whereas in developed countries widespread cytology-based screening has led to a sharp decline in incidence [1, 3].
The main risk factor is chronic human papillomavirus (HPV) infection [1, 3]. Thus, cervical cancer can be effectively prevented with HPV vaccines and HPV DNA-based screening [1]. Abnormal cytology or positive high-risk HPV test should prompt colposcopy and cervical biopsy. Squamous cell carcinoma comprises 70–80% and adenocarcinoma 20–25% of cases [1].
18.1.2 Initial Staging and Management
Cervical cancer is staged using the International Federation of Gynecology and Obstetrics (FIGO) classification [4]. It is the only gynecologic malignancy staged clinically owing to high prevalence in developing countries. Lymph node (LN) status, a major prognostic factor, is excluded from FIGO staging as clinical detection is not possible [1]. Cross-sectional imaging is not accepted for formal assignment of FIGO stage but is used routinely in developed countries to guide treatment decisions [1, 5]. Treatment selection is determined by tumor stage and presence/location of LN metastases (Fig. 18.1).
Diagram (adapted from [6]) highlights the role of MRI and PET/CT to assess disease extent and guide treatment decisions in patients with new diagnosis of cervical cancer
18.1.3 MRI and Initial Staging
MRI is the method of choice to evaluate locoregional extent (Table 18.1) [6, 7]. Small field-of-view T2-weighted imaging in the sagittal and short-axis planes is the mainstay of staging MRI (Fig. 18.2) [6]. Diffusion-weighted imaging (DWI) in conjunction with T2-weighted imaging improves delineation of tumor margins. Dynamic contrast-enhanced imaging (DCE) is used largely in research for prognosis and response assessment.
Drawing illustrates orientations of short-axis planes to stage endometrial cancer (solid line oriented perpendicular to endometrium) and cervical cancer (dashed line oriented perpendicular to endocervical canal). Double line is drawn at the bladder base/urethral junction and divides the vagina into upper and lower portions
18.1.3.1 Stage I: Cervix-Confined Disease
Stage IA is microscopic in size. Stage IB is visible clinically and is divided based on size into IB1 (≤4 cm) and IB2 (>4 cm). The tumor has intermediate signal intensity (SI) on T2-weighted imaging and shows diffusion restriction. Intact rim of low-SI fibrous cervical stroma around the tumor indicates cervix-confined disease (Fig. 18.3) [6]. Fertility preservation (LN assessment + cone/trachelectomy) is possible for patients with ≤Stage IB1 (Table 18.2) [8, 9].
52-year-old woman with Stage IB1 cervical adenocarcinoma. Sagittal T2-weighted imaging (a) and short-axis T2-weighted imaging (b) demonstrate 1.8 cm tumor with intermediate SI on T2-weighted imaging. Tumor involves partial thickness of cervical stroma with intact low-SI stromal ring (arrow) ruling out parametrial invasion
18.1.3.2 Stage II: Cervical + Upper Vaginal Disease ± Parametrial Invasion
Stage IIA invades the upper vagina (upper two-thirds) but not the parametrium. Similar to Stage IB, it is divided into IIA1 (≤4 cm) and IIA2 (>4 cm). Vaginal invasion is diagnosed when intermediate-SI tumor disrupts low-SI vagina on T2-weighted imaging. A horizontal line drawn at the bladder base on sagittal T2-weighted imaging divides the vagina into upper and lower portions (Fig. 18.2).
Stage IIB invades the parametrium. Complete loss of low-T2 SI stromal ring does not always indicate parametrial invasion. Parametrial invasion is present if tumor disrupts low-SI stroma AND causes spiculated/nodular tumor-parametrial interface ± encases parametrial vessels (Fig. 18.4) [6]. DWI + T2-weighted imaging improve specificity for detecting parametrial invasion [10]. Accurate diagnosis is important as parametrial invasion indicates concurrent chemotherapy and radiation (CCRT) as treatment of choice (Fig. 18.1).
35-year-old woman with Stage IIB cervical squamous cell carcinoma. Sagittal T2-weighted imaging (a), short-axis T2-weighted imaging (b), and fused T2-weighted imaging + DWI (c) demonstrate 5.2 cm tumor involving the lower uterus, upper vagina (arrow), and parametrial regions. There is nodular interface between the tumor and parametrium (arrow). Also note right internal iliac LNs (arrowhead)
18.1.3.3 Stage III: Pelvic Sidewall Invasion, Lower Vaginal Involvement, Hydronephrosis, or Nonfunctional Kidney
Stage IIIA invades the lower vagina. Stage IIIB extends to the pelvic sidewall ± causes hydronephrosis. Tumor within 3 mm of iliac vessels or obturator internus, piriformis, or levator ani muscles indicates sidewall invasion [6].
18.1.3.4 Stage IV: Bladder/Rectal Mucosal Invasion or Extension Beyond the Pelvis
Stage IVA invades the bladder/rectal mucosa; invasion is present when intermediate-SI tumor disrupts low-SI muscle wall and extends into the mucosa on T2-weighted imaging. Bullous edema alone (high-SI mucosa) does not mean Stage IVA [6]. Stage IVB is indicated by the presence of distant metastases (including LN metastases beyond the pelvis).
18.1.4 PET/CT and Initial Staging
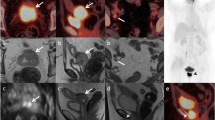
LN metastases impact prognosis and treatment. On MRI (and CT) short-axis diameter ≥ 1 cm is a main diagnostic criterion but yields low-to-moderate sensitivity because metastases to normal-sized LNs remain undetected (Fig. 18.5) [11]. LNs show high SI on DWI, increasing their conspicuity (Fig. 18.4); however, DWI is not used to distinguish benign from malignant LNs due to overlap in ADC values [10]. PET/CT improves N and M staging and is recommended for Stage ≥ IB2 disease (Fig. 18.5) [5, 12].
Same patient as in Fig. 18.4. Axial T2-weighted imaging and PET/CT images show 0.5 cm short-axis FDG-avid left para-aortic LN (a, b). (c) Baseline axial PET/CT image through the pelvis demonstrates FDG-avid cervical tumor and right internal iliac LN (arrow). (d) Complete resolution of FDG avidity after CCRT
18.1.5 Evaluation During and After Initial Treatment
After radical hysterectomy, imaging is obtained if recurrence is suspected clinically [1, 5]. After fertility-sparing surgery, MRI is recommended at 6 months and annually thereafter [5].
MRI and PET/CT are useful for radiotherapy (RT) planning. For example, RT field is extended to para-aortic regions if para-aortic LN metastases are present on pretreatment PET/CT (Figs. 18.1 and 18.5) [1, 5], and mid-treatment MRI allows adjustment of brachytherapy dose based on volume/location of residual tumor [13, 14].
Low-SI stroma on T2-weighted imaging indicates tumor-free cervix, but an abnormal signal may persist up to 6 months after CCRT [6]. Thus, PET/CT obtained 3–6 months after CCRT is useful. Absent FDG avidity indicates low risk of recurrence; diminished FDG avidity signifies moderate risk; and unchanged, increased, or new FDG-avid foci indicate persistent disease (Fig. 18.5) [15]. The role of DWI and DCE to assess response is an area of active research [10, 13].
18.1.6 Evaluation of Recurrence
Recurrent cervical cancer has similar imaging features as primary lesions [6]. In the absence of prior pelvic RT, localized recurrence may be approached with CCRT. Radical surgery is the only potential cure in previously irradiated pelvis. MRI and PET/CT are essential to determine eligibility and tailor the extent of surgery [16].
18.1.7 Future Directions
PET/MRI may offer a “one-stop-shop” approach to the evaluation of cervical cancer in both primary and recurrent settings [17].
18.2 Endometrial Cancer
18.2.1 Epidemiology and Diagnosis
Endometrial cancer is the sixth most common malignancy among women worldwide and the most common gynecological cancer in developed countries [2]. Incidence has steadily increased, primarily due to increased longevity and obesity in developed countries. Approximately 75% of cases occur in postmenopausal women and >90% present with postmenopausal bleeding. As such, most patients are diagnosed at an early stage with 5-year survival rates >90% in this subgroup [18].
Initial evaluation includes pelvic ultrasound (US) and pipelle endometrial biopsy or dilatation and curettage. Thresholds of endometrial thickness of 4–5 mm in postmenopausal women detect endometrial cancer with a sensitivity of up to 95% and a specificity of 77% [19]. Endometrial cancer is traditionally subdivided into two types based on biological behavior: type 1 tumors are estrogen-dependent and include FIGO grades 1 and 2 endometrioid adenocarcinomas, while type 2 tumors include serous papillary carcinomas, clear cell adenocarcinomas, carcinosarcomas, and FIGO grade 3 endometrioid adenocarcinomas [18]. Type 2 tumors are not estrogen dependent, present at a higher stage, and behave more aggressively.
18.2.2 Initial Staging and Management
Endometrial cancer is staged surgically using the FIGO system [4]. The standard surgical staging procedure consists of total abdominal hysterectomy, bilateral salpingo-oophorectomy, LN dissection, peritoneal washing, and omental biopsies. FIGO stage correlates well with prognosis, but preoperative staging remains important, since early-stage/low-grade tumors may be treated with minimally invasive surgery and may not require LN dissection [20, 21].
18.2.3 MRI and Initial Staging
MR imaging is the modality of choice to determine the depth of myometrial invasion, which correlates with tumor grade, LN metastases, and 5-year survival (Table 18.1) [22]. Small field-of-view high-resolution imaging in the sagittal and short-axis planes is the mainstay of MRI staging (Fig. 18.2) [6]. The combination of T2-weighted imaging, DCE, and DWI offers the best diagnostic accuracy [23, 24].
MRI with its high-spatial resolution and larger field of view has a particular advantage over endovaginal US in the setting of bulky tumors, advanced stage, and concomitant benign disease, i.e., adenomyosis and LMs.
18.2.3.1 Stage I: Uterine-Confined Disease
Stage IA is confined to the endometrium or inner half of the myometrium, i.e., <50% myometrial thickness involvement (Fig. 18.6). Stage IB extends to the outer half of the myometrium (≥50%) (Fig. 18.7). The tumor has intermediate-to-low SI on T2-weighted imaging, is hypointense to myometrium on later phases of DCE, and shows restricted diffusion. However, small tumors may be iso- or only minimally hypointense relative to the normal endometrium and may be hypervascular relative to the endometrium early on after contrast administration. A partially intact, low-SI junctional zone around the tumor indicates the absence of deep myometrial invasion.
62-year-old woman with Stage IA endometrioid adenocarcinoma (grade 2) of the endometrium. Short-axis (a) T2-weighted imaging, (b) B1000 DWI, and (c) delayed post gadolinium 3D T1-weighted imaging demonstrate an endometrial-based tumor (thick arrow) at the level of the fundus and right cornua. There is partial invasion of the inner half of the myometrium with a thin rim of junctional zone (thin arrow) remaining intact
76-year-old woman with Stage IB endometrioid adenocarcinoma (grade 3) of the endometrium. Short-axis (a) T2-weighted imaging, (b) B1000 DWI, and (c) delayed post gadolinium 3D T1-weighted imaging demonstrate a large tumor (thick arrow) invading the deep myometrium. There is a thin rim of intact outer myometrium (thin arrow) along the left aspect of the uterus excluding serosal involvement
18.2.3.2 Stage II: Cervical Stromal Invasion
Stage II invades and disrupts the low-SI cervical stroma on T2-weighted imaging. Polypoid extension of the tumor into the endocervical canal or endocervical mucosal invasion is not sufficient to assign Stage II. Tumor extension beyond the cervix into the parametrium requires a radical hysterectomy.
18.2.3.3 Stage III: Invasion of Uterine Serosa, Adnexa, Vagina, and/or Pelvic or Para-Aortic Lymph Nodes
Stage IIIA invades the uterine serosa appearing as an area of intermediate SI on T2-weighted imaging with loss of normal rim enhancement of the outer myometrium on DCE. Direct tumor spread to the adnexa or ovarian metastases is considered Stage IIIA disease. It is important but challenging to differentiate synchronous ovarian lesions from metastases to the ovaries. Type 2 tumors have a higher pretest probability of advanced disease, and therefore a meticulous search for advanced tumor extent is required in this subgroup.
Stage IIIB involves the vagina either by contiguous spread or metastatic involvement.
Stage IIIC is diagnosed when there is pelvic (Stage IIIC1) and/or para-aortic (Stage IIIC2) LN metastatic involvement. The use of a 1 cm short-axis cutoff value results in a high specificity but poor sensitivity (range, 36–89.5%) for predicting LN metastases [6]. A cutoff value of 0.8 cm increases the sensitivity albeit at the cost of specificity. In practice, given that endometrial carcinoma is staged surgically, it is important to provide the size and detail the location of LNs exceeding 0.5–0.6 cm in short-axis. The role of DWI and ADC to assess LNs is the same as described for cervical cancer [10]. PET/CT improves N and M staging; however, PET/CT is currently not part of standard of care for initial staging of endometrial cancer.
18.2.3.4 Stage IV: Bladder/Rectal Mucosal Invasion or Extension Beyond the Pelvis
Please refer to section “MRI and Initial Staging of Cervical Carcinoma,” as the findings are the same for endometrial and cervical carcinoma.
18.2.4 Evaluation of Recurrence
Recurrent endometrial cancer has a similar imaging appearance as the primary tumor. Risk factors for recurrence include advanced stage at presentation, high-grade disease, and lymphovascular invasion. More than 80% of recurrences occur within 3 years of initial treatment with LNs (46%) and the vaginal vault (42%) being the most common sites. MRI is useful to evaluate surgical resectability of pelvis-confined recurrent disease, while PET/CT is helpful to exclude distant and LN metastases [6, 16].
18.3 Uterine Sarcomas
18.3.1 Epidemiology and Presentation
Uterine sarcomas are rare aggressive tumors originating from mesenchyme and comprising <3% of uterine corpus tumors [6, 21]. Leiomyosarcoma (LMS) is the most common histologic subtype, followed by endometrial stromal sarcoma (ESS) and undifferentiated uterine sarcoma. ESS is subdivided into low- and high-grade ESS based on differences in histopathology and clinical outcomes [21]. Carcinosarcoma was previously categorized as uterine sarcoma but is now classified as high-grade endometrial carcinoma [6, 21].
Uterine sarcomas arise de novo and have no biologic link to LM [25]. Prompt radical surgery is the standard of care for uterine sarcomas [21]. In contrast, symptomatic LM can be approached with traditional surgery (hysterectomy, myomectomy) or newer minimally/noninvasive strategies (embolization, ablation, medical therapy). The latter do not yield histologic diagnosis, highlighting the need for precise initial diagnosis [26].
Uterine sarcoma and LM manifest with similar symptoms. Large size and rapid growth are unreliable signs of malignancy [25]. Growth of uterine mass after menopause and elevated LDH, particularly LDH isozyme type 3, should be viewed as suspicious for LMS [25]. Endometrial sampling may aid diagnosis of uterine sarcoma, but sensitivity is limited due to myometrial origin [21, 25].
18.3.2 Role of Imaging
MRI is the imaging method of choice if uterine sarcoma is suspected clinically or if intervention is planned for symptomatic LM (to map tumors and exclude sarcoma) [26].
No single MR feature can reliably distinguish uterine sarcoma (including LMS) from atypical LM (degeneration or histologic variants). Combined MR features may suggest correct diagnosis including a single large uterine mass with hemorrhage (high SI on T1-weighted imaging), heterogeneous high SI on T2-weighted imaging, infiltrative/nodular borders on T2-weighted imaging, and central necrosis [27]. Uterine sarcoma also demonstrates rapid early enhancement of solid components on DCE and restricted diffusion (Fig. 18.8) [27]. Diffusion restriction alone is insufficient for diagnosis because it may be observed with LM, especially cellular LM [27].
ESS often demonstrates distinct MR features that aid diagnosis including an ill-defined myometrial mass with endometrial component, wormlike low-SI bands on T2-weighted imaging (normal myometrium compressed by tumor), and contiguous extension along the fallopian tubes, periuterine vessels, and pelvic ligaments [6].
Key Points
-
MRI excels at locoregional staging of cervical and endometrial cancer for treatment stratification, while PET/CT improves N and M staging in uterine malignancies.
-
MRI facilitates patient selection prior to fertility preservation for early stage cervical and endometrial cancers.
-
MRI aids the characterization of clinically suspected uterine sarcoma.
References
Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv72–83.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Ginsburg O, Bray F, Coleman MP, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389:847–60.
Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Cervical Cancer. Version 1.2017. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed 1 July 2017.
Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266:717–40.
Raithatha A, Papadopoulou I, Stewart V, Barwick TD, Rockall AG, Bharwani N. Cervical cancer staging: a Resident’s primer: Women’s imaging. Radiographics. 2016;36:933–4.
Bentivegna E, Gouy S, Maulard A, Chargari C, Leary A, Morice P. Oncological outcomes after fertility-sparing surgery for cervical cancer: a systematic review. Lancet Oncol. 2016;17:e240–53.
Rockall AG, Qureshi M, Papadopoulou I, et al. Role of imaging in fertility-sparing treatment of gynecologic malignancies. Radiographics. 2016;36:2214–33.
deSouza NM, Rockall A, Freeman S. Functional MR imaging in gynecologic cancer. Magn Reson Imaging Clin N Am. 2016;24:205–22.
Choi HJ, Ju W, Myung SK, Kim Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci. 2010;101:1471–9.
Signorelli M, Guerra L, Montanelli L, et al. Preoperative staging of cervical cancer: is 18-FDG-PET/CT really effective in patients with early stage disease? Gynecol Oncol. 2011;123:236–40.
Khan SR, Rockall AG, Barwick TD. Molecular imaging in cervical cancer. Q J Nucl Med Mol Imaging. 2016;60:77–92.
Potter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–23.
Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–95.
Lakhman Y, Nougaret S, Micco M, et al. Role of MR imaging and FDG PET/CT in selection and follow-up of patients treated with pelvic Exenteration for gynecologic malignancies. Radiographics. 2015;35:1295–313.
Bagade S, Fowler KJ, Schwarz JK, Grigsby PW, Dehdashti F. PET/MRI evaluation of gynecologic malignancies and prostate cancer. Semin Nucl Med. 2015;45:293–303.
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–108.
Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510–7.
Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Uterine Neoplasms. Version 3.2017. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed 15 Oct 2017.
Kinkel K, Forstner R, Danza FM, et al. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol. 2009;19:1565–74.
Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S. MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. Eur Radiol. 2014;24:1327–38.
Deng L, Wang QP, Chen X, Duan XY, Wang W, Guo YM. The combination of diffusion- and T2-weighted imaging in predicting deep Myometrial invasion of endometrial cancer: a systematic review and meta-analysis. J Comput Assist Tomogr. 2015;39:661–73.
Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208–16.
Silberzweig JE, Powell DK, Matsumoto AH, Spies JB. Management of Uterine Fibroids: a focus on uterine-sparing interventional techniques. Radiology. 2016;280:675–92.
Bolan C, Caserta MP. MR imaging of atypical fibroids. Abdom Radiol. 2016;41:2332–49.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Lakhman, Y., Reinhold, C. (2018). Malignant Diseases of the Uterus. In: Hodler, J., Kubik-Huch, R., von Schulthess, G. (eds) Diseases of the Abdomen and Pelvis 2018-2021. IDKD Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-319-75019-4_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-75019-4_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75018-7
Online ISBN: 978-3-319-75019-4
eBook Packages: MedicineMedicine (R0)