Abstract
The paper presents the results of studies aimed at obtaining experimental data for development of technological solutions to the production of marketable petrochemicals and petroleum products from heavy oil wastes using the process of hydroconversion and at designing engineering solutions in the area of preliminary treatment of heavy oil wastes for their further processing into marketable petrochemicals and petroleum products. The experiments have been conducted using a bench for heavy oil waste processing which combines pretreatment of oil wastes and subsequent hydroconversion processing in the presence of highly efficient ultrafine catalysts. Optimum conditions of oil sludge heavy residue hydroconversion (pressure, 7 MPa; temperature, 435 ℃; feedstock space velocity, 1 h−1; hydrogen: feedstock, 1000 nL/L; catalyst (Mo) content, 0.05 wt%; H2O, 2 wt% (based on the feedstock) make it possible to achieve conversion of the 520 ℃+ fraction of feedstock of up to 67 wt% (per pass) or 90 wt% (based on the recycle stock).
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Environmental control
- Petroleum residues
- Sludges
- Heavy residue
- Heavy oil feedstock
- Hydrogen
- Oil-Refining products
- Hydroconversion
- Ultrafine catalyst
Production, transportation, and refining of oil feedstock are inevitably associated with the appearance of diverse oil wastes and lead to considerable environmental pollution by marked amounts of heavy wastes such as sludge of various origin, vacuum residue, heavy residual fractions, still bottoms, residues in oil storage tanks, etc. [1,2,3,4,5]. These wastes are formed both in industrial controlled processes, such as oil refining from water, treatment of oil-containing effluents in treating facilities, during oil storage and transportation in various tanks and in emergencies with oil spillage.
Accumulation of oil wastes causes substantial pollution of the environment and involves the concentration of substantial environmental damage (it is believed that about 1 t of sludge is generated per 500 t of oil; this value is valid for developed countries). Disposal in sludge collectors, which are open earth capacities for sludge storage and which occupy vast surface areas, leads to alienation of agricultural lands and environmental pollution because of evaporation of petroleum products and their penetration into ground waters. Heavy aromatic hydrocarbons in sludges exhibit well-defined carcinogenic and mutagenic properties. The sludges and wastes are highly resistant to decomposition in the environment, and their components may be distributed over marked distances, being accumulated in animals, plants, soil, and water, thereby destroying the equilibrium of environmental systems and leading to the death of animals and plants and thus making environment unfit for life. Penetrating into the human body, these compounds accumulate in fat tissues and cause genetic mutations and keratosis of newborns. As a consequence, the neutralization and disposal of oil sludges is a pressing issue [5, 6].
Oil wastes are as rule heavy oil residues. The presence of water and solid impurities in them and the predominance of heavy asphaltic and resinous hydrocarbons and the products of physicochemical interaction of petroleum or petroleum products with oxygen, moisture, and mechanical impurities noticeably impede their qualified use [7,8,9].
Methods available for the disposal of oil sludges [10,11,12,13,14,15,16,17,18] do not often provide the necessary level of environmental protection against secondary pollutions and do not permit the efficient use of these resources. Modern approaches to the processing of oil wastes should envisage refusal not only of disposal (which is inapplicable from the point of view of environmental protection) but also of combustion. The processing of oil wastes should be directed at the recovery of the organic part of wastes and its subsequent processing. The most qualified method for processing of the organic part of heavy oil wastes is their chemical processing via hydroconversion [15]. This approach, on one hand, preserves the chemical potential of hydrocarbon components of heavy oil wastes and, on the other hand, makes it possible to transform carbonaceous components of oils wastes into motor fuels, thereby increasing the total depth of petroleum processing.
The authors of this paper propose a new approach to the processing of heavy oil wastes. The essence of this approach consists in the initial isolation of the organic part of oil wastes using a complex of pretreatment methods (extraction, filtration, distillation) and further hydroconversion processing of heavy hydrocarbon residue of oil wastes in the presence of highly efficient ultrafine catalysts that provide a high yield of distillate fractions.
For this purpose, a complex experimental bench for hydroconversion processing of heavy oil wastes was designed which allows pretreatment of oil wastes and their subsequent processing by the hydroconversion technology in the presence of highly efficient ultrafine catalysts.
The goal of this study is to obtain the experimental data for the development of technological solutions to the production of marketable petrochemicals and petroleum products from heavy oil wastes via the hydroconversion process and for designing engineering solutions in the area of pretreatment of heavy oil wastes for their further processing into marketable petrochemicals and petroleum products.
Experimental
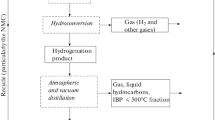
Experiments on the processing of heavy oil wastes were performed on a complex experimental bench for the hydroconversion processing of heavy oil wastes (HPW) (Fig. 1). The bench is composed of an HPW pretreatment unit, an atmospheric–vacuum distillation unit, and a hydroconversion unit. The HPW pretreatment unit consists of an extractor, a settler, and a filter. The hydroconversion unit contains units of feedstock pretreatment and the introduction of additional components, a reactor unit, and a unit for separating target products.
Heavy residue of the organic part of oil tank sludge (HRS) and vacuum residue of West-Siberian oil refining was used as heavy oil wastes. HRS was obtained in the pretreatment unit of the complex experimental hydroconversion bench as described in [19].
Physicochemical properties of the feedstock and hydroconversion products were studied as described in [20].
Ammonium paramolybdate (NH4)6Mo7O24·4H2O (APM) was used as a catalyst precursor. Before feeding in the reactor, the feed emulsion was preliminarily prepared, in which the dispersion medium and the dispersed phase were heavy oil waste and APM aqueous solution, respectively. The load of the APM aqueous solution with respect to feedstock was 0.05 wt% (based on Mo) and 2 wt% water. The efficiency of hydroconversion was estimated from the degree of conversion of feedstock fraction boiling above 520 ℃ (in what follows, 520 ℃+) and from coke deposition on reactor walls (Table 1).
Discussion of Applied Results
Our experiments on the hydroconversion of vacuum residue showed that optimum conditions for the hydroconversion of West-Siberian oil vacuum residue are as follows: pressure in a reactor, 7 MPa; temperature in the reactor, 440 ℃; hydrogen: feedstock, 1000 nL/L; feedstock space velocity, 1 h−1; and the content of molybdenum in the reaction zone, 0.05 wt%. Under these conditions, the conversion of the 520 ℃ + fraction attains 55.5% at a coke yield of 0.05%.
The heavy residue of oil sludge contains a smaller amount of asphaltic and resinous hydrocarbons than the vacuum residue while the amount of paraffin-naphthenic hydrocarbons is higher than that in vacuum residue. Therefore, the hydroconversion of HRS should proceed more easily than the hydroconversion of vacuum residue. This fact made it possible to assume optimum conditions of vacuum residue hydroconversion (the maximum level of conversion at the minimum level of coking) as initial for the study of HRS hydroconversion.
Research into the effect of temperature on the hydroconversion of HRS (Table 2) revealed that the optimum conditions of HRS processing are as follows: a pressure of 7 MPa, a temperature of 435 ℃, a feedstock space velocity of 1 h−1, hydrogen: HRS, 1000 nL/L, and aqueous solution of ammonium paramolybdate as a catalyst precursor (Mo, 0.05 wt%; H2O, 2 wt% based on the feedstock).
Under the optimum conditions of HRS hydroconversion, the conversion of the 520 ℃ + fraction is 67 wt%. In order to increase the depth of feedstock conversion, it is advisable to return heavy distillation residue (recycle stock) containing the 520 ℃ + fraction which was isolated from the hydrogenation product to the hydroconversion of vacuum residue. This trick makes it possible not only increase the final degree of conversion but also to reduce consumption of the fresh catalyst due to recycling. Conversions with the added recycle stock are calculated under the assumption that the 520 ℃ + fraction in the recycle stock constantly occurs in the system and enters into the 520 ℃ + fraction of the hydrogenation product.
The total conversion may be calculated via the following formula:
where C0 is the amount of the 520 ℃ + fraction in the fresh feedstock (vacuum residue); Cp is the amount of the 520 ℃ + fraction; and C1 is the amount of the 520 ℃ + fraction after hydroconversion.
The experimental studies, in this case, include the hydroconversion of HRS, atmospheric-vacuum distillation of the hydrogenation product, mixing of the obtained residue of hydrogenation product distillation, that is, the recycle stock containing the active catalyst, with vacuum residue, and introduction of the deficient amount of the precursor in the form of aqueous solution followed by mixture dispersing to obtain a molybdenum concentration in the reaction mixture of 0.05%. The properties of HRS obtained under the optimum conditions of hydroconversion are summarized in Table 3.
In the hydroconversion of HRS with the addition of the recycle stock, the conversion of the 520 ℃ + fraction was 90.2 wt% (Table 4) with a high yield of marketable distillate fractions.
Conclusions
It has been shown that the optimum conditions of hydroconversion of oil sludge heavy residue (pressure, 7 MPa; temperature, 435 ℃; feedstock space velocity, 1 h−1; hydrogen: feedstock, 1000 nL/L; catalyst (Mo) content, 0.05 wt%; H2O, 2 wt% (based on the taken feedstock)) make it possible to attain a conversion of the 520 ℃+ fraction of the feedstock of up to 67 wt% (per pass) and up to 90 wt% (based on the recycle stock). The above experimental data may be used as a basis for the development of technological solutions to a new competitive method of heavy oil waste processing to marketable petrochemicals via hydroconversion conducted in the presence of highly efficient ultrafine catalysts.
References
Shperber, E.R.: Some kinds of wastes of oil refineries and their classification. Environ. Prot. Oil-And-Gas Indus. 2, 27–32 (2011)
Hu, G., Li, J., Zeng, G.: Recent development in the treatment of oily sludge from petroleum industry: a review. J. Hazard. Mater. 261, 470–490 (2013)
Elektorowicz, M., Habibi, S.: Sustainable waste management: recovery of fuels from petroleum sludge. Can. J. Civil. Eng. 32, 164–169 (2005)
Shailubhai, K.: Treatment of petroleum industry oil sludge in soil. Trends Biotechnol. (1986)
Treatment of refinery wastes (oil sludge). Oil Gas J. 89(l), 73–77 (1991)
Gron’, V.A., Korostovenko, V.V., Shakhrai, S.G., Kaplichenko, N.M., Galaiko, A.V.: Problems of generation, processing, and disposal of oil sludges. Adv. Mod. Nat. Sci. 9, 159–162 (2013)
Ulistkii, V.A.,Vasil’vistkii, A.E., Plushchevskii, M.B.: Industrial Wastes and Resource Saving. In: Sashko, M. (ed.) 368 pp (2006)
Mazlova, E.A., Meshcheryakov, S.V.: Oil sludge disposal and reclaiming problems. M.: Noosphere, 56 pp (2001)
Minigazimov, N.S., Pasvetalov, V.A.: Disposal and Treatment of Oil-Containing Wastes, p. 299. Ekologiya, Ufa (1999)
Shlepkina, Yu.S.: Analysis of methods for disposal of oil sludges. Advantages and drawback. Environ. prot. oil-and-gas ind. 32–34 (2009)
Velghe, I., Carleer, R., Yperman, J., Schreurs, S.: Study of the pyrolysis of sludge and sludge/disposal filter cake mix for the production of value added products. Bioresour. Technol. 134, 1 (2013)
Shie, J., Lin, J., Chang, C., Wu, C., Lee, D., Chang, C., Chen, Y.: Oxidative thermal treatment of oil sludge at low heating rates. Energ. Fuels 18, 1272 (2004)
Zheng, C., Wang, M., Wang, Y., Huang, Z.: Optimization of biosurfactant-mediated oil extraction from oil sludge. Bioresour. Technol. 110, 338 (2012)
Je-Lueng, Shie, Jyh-Ping, Lin, Ching-Yuan, Chang, Shin-Min, Shih, Duu-Jong, Lee, Chao-Hsiung, Wu: Pyrolysis of oil sludge with additives of catalytic solid wastes. J. Anal. Appl. Pyrolysis 71(2), 70–695 (2004)
Rocha, O., Dantas, R., Duarte, M., Silva, V.: Oil sludge treatment by photocatalysis applying black and white light. Chem. Eng. J. 157, 80–85 (2010)
Xu, N., Wang, W., Han, P., Lu, X.: Effects of ultrasound on oily sludge deoiling. J. Hazard. Mater. 171, 914–917 (2009)
Roldán-Carrillo, T., Castorena-Cortés, G., Zapata-Peñasco, I., Reyes-Avila, J., Olguín-Lora, P.: Aerobic biodegradation of sludge with high hydrocarbon content generated by a mexican natural gas processing facility. J. Environ. Manage. 95, 93–98 (2012)
Kadiev, Kh., Dandaev, A.,Gyul’Maliev, A., Batov, A., Khadzhiev, S.: Hydroconversion of Polyethylene and tire rubber in a mixture with heavy oil residues. Solid Fuel Chem. 47(2), 132–138 (2013)
Kadiev, Kh., Kadieva, M., Batov, A., Dandaev, A., Oknina, N., Maksimov, A.: Studies on preprocessing of reservoir oil sludges for further hydroconversion. Biosci. Biotechnol. Res. Asia 12 (Spl. Ed. 2), 473–483 (2015)
Oknina, N., Kadiev, Kh., Kadieva, M., Maksimov, A., Batov, A., Dandaev, A.: Physico-Chemical properties of oil sludges from reservoirs. Biosci. Biotechnol. Res. Asia 12 (Spl. Ed. 2), 497–505 (2015)
Acknowledgments
Research was supported by the Ministry of Education and Science of the Russian Federation. Agreement (contract) no. № 14.579.21.0052 22.09.2014. Unique Project Identifier: RFMEFI57914X0052.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2018 The Author(s)
About this paper
Cite this paper
Batov, A.E., Kadiev, K.M., Kadieva, M.K., Maximov, A.L., Oknina, N.V. (2018). Hydrogenation Processing of Heavy Oil Wastes in the Presence of Highly Efficient Ultrafine Catalysts. In: Anisimov, K., et al. Proceedings of the Scientific-Practical Conference "Research and Development - 2016". Springer, Cham. https://doi.org/10.1007/978-3-319-62870-7_69
Download citation
DOI: https://doi.org/10.1007/978-3-319-62870-7_69
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62869-1
Online ISBN: 978-3-319-62870-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)