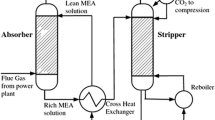
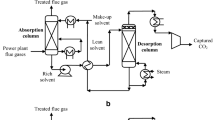
Abstract
Post-combustion CO2 capture (PCC) is considered the most feasible and viable process for CO2 abatement in the power sector. Aqueous monoethanolamine (MEA) solvent, traditionally used in this process, brings along challenges, namely, huge energy requirement for solvent regeneration, huge solvent flow rate leading to large equipment sizes, and chemical and thermal degradability, among others. In this study, the prospects of replacing aqueous MEA solvent with a blend of ionic liquid (IL) and MEA are explored. IL is generally chemically and thermally stable among other encouraging properties but is however expensive. A blend of IL and MEA is predicted to have shared qualities of MEA and IL and therefore could hypothetically contribute to meaningful reduction in overall cost of the process.
This hypothesis is investigated in this study by performing a technical and economic analysis of the process using aqueous blend of IL ([Bpy][BF4]) and MEA as solvent. A rate-based model of the process developed in Aspen Plus was used to perform the technical and economic studies. Technical and economic analysis of PCC with aqueous blend of IL and MEA as solvent have not been covered in existing studies. Also, reported models are derived using equilibrium-based approach.
From the analysis, it is found that with about 5 wt% IL concentration, total solvent cost approximates closely to typical solvent cost for the MEA only process; higher IL concentration leads to significant increase in solvent cost. Also, the simulation results showed that the rate-based [Bpy][BF4]-MEA process can save about 7–9% regeneration heat duty and reduce the solvent flow rate by about 11.5–27% compared to the conventional MEA only process.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Reference
Aboudheir, A., Tontiwachwuthikul, P., Chakma, A.: Kinetics of the reactive absorption of carbon dioxide in high CO2-loaded, concentrated aqueous monoethanolamine solutions. Chem. Eng. Sci. 58, 5195–5210 (2003)
Bravo, J.L., Rocha, J.A., Fair, J.R.: Hydrocarbon Process, p. 91 (1985)
Bravo, J.L., Rocha, J.A., Fair, J.R.: A Comprehensive Model in the Performance of Columns Containing Structured Packings, Distillation and Absorption, Institution of Chemical Engineers Symposium Series 128, p. 1. Institution of Chemical Engineers (1992)
Camper, D., Bara, J., Gin, D.L., Noble, R.: Room-temperature ionic liquid-amine solutions: tunable solvents for efficient and reversible capture of CO2. Ind. Eng. Chem. Res. 47, 8496–8498 (2008)
Canepa, R., Wang, M., Biliyok, C., Satta, A.: Thermodynamic analysis of combined cycle gas turbine power plant with post-combustion CO2 capture and exhaust gas recirculation. J Process Mech Eng. 227(2), 89–105 (2012)
Davidson, R.M.: Post-combustion carbon capture from coal fired plants – solvent scrubbing. IEA Clean Coal Centre, CCC/125 (2007)
Hasib-ur-Rahman, M., Bouteldja, H., Fongarland, P., Siaj, M., Larachi, F.: Corrosion behaviour of carbon steel in alkanolamine/room-temperature ionic liquid based CO2 capture system. Ind. Eng. Chem. Res. 51, 8711–8718 (2012)
Huang, Y., Zhang, X., Zhang, X., Dong, H., Zhang, S.: Thermodynamic modeling and assessment of ionic liquid-based CO2 capture processes. Ind. Eng. Chem. Res. 53, 11805–11817 (2014)
Jassim, M.S., Rochelle, G.T.: Innovative absorber/desorber configurations for CO2 capture by aqueous monoethanolamine. Ind. Eng. Chem. Res. 45, 2465–2472 (2006)
Lawal, A., Wang, M., Stephenson, P., Yeung, H.: Dynamic modelling of CO2 absorption for post combustion capture in coal-fired power plans. Fuel. 88, 2455–2462 (2009)
Lawal, A., Wang, M., Stephenson, P., Obi, O.: Demonstrating full-scale post-combustion CO2 capture for coal-fired power plants through dynamic modelling and simulation. Fuel. 101, 115–128 (2012)
Nataly Echevarria Huaman, R., Xiu Jun, T.: Energy related CO2 emissions and the progress on CCS projects: a review. Renew. Sust. Energ. Rev. 31, 368–385 (2014)
Onda, K., Takeuchi, H., Okumoto, Y.: Mass transfer coefficients between gas and liquid phases in packed columns. Journal of Chemical Engineering of Japan. 1, 56–62 (1968)
Peng, J., Edgar, T.F., Eldridge, R.B.: Dynamic rate-based and equilibrium models for a packed reactive distillation column. Chem. Eng. Sci. 58, 2671–2680 (2003)
Ramdin, M., de Loos, T.W., Vlugt, T.J.H.: State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 51, 8149–8177 (2012)
Shiflett, M.B., Drew, D.W., Cantini, R.A., Yokozeki, A.: Carbon dioxide capture using ionic liquid 1-butyl-3-methylimidazolium acetate. Energy Fuel. 24, 5781–5789 (2010)
Stichlmair, J., Bravo, J.L., Fair, J.R.: General model for prediction of pressure drop and capacity of countercurrent gas/liquid packed columns. Gas Separation and Purification. 3(1), 19–28 (1989)
Wappel, D., Gronald, G., Kalb, R., Draxler, J.: Ionic liquids for post-combustion CO2 absorption. International Journal of Greenhouse Gas Control. (2010)
Yang, J., Yu, X., Yan, J., Tu, S.: CO2 capture using amine solution mixed with ionic liquid. Ind. Eng. Chem. Res. 53, 2790–2799 (2014)
Yu, G., Zhang, S., Zhou, G., Liu, X., Chen, X.: Structure, interaction and property of amino-functionalized imidazolium ILs by molecular dynamics simulation and ab initio calculation. Appl. Energy. 53, 3210–3221 (2007)
Zhang, H., Rubin, E.S.: Systems analysis of ionic liquids for post-combustion CO2 capture at coal-fired power plants. Energy Procedia. 63, 1321–1328 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Zacchello, B., Oko, E., Wang, M., Aloui, F. (2018). Technical and Economic Analysis of Ionic Liquid-Based Post-combustion CO2 Capture Process. In: Aloui, F., Dincer, I. (eds) Exergy for A Better Environment and Improved Sustainability 1. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-62572-0_89
Download citation
DOI: https://doi.org/10.1007/978-3-319-62572-0_89
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62571-3
Online ISBN: 978-3-319-62572-0
eBook Packages: EnergyEnergy (R0)