Abstract
Eosinophils are granular leukocytes known to have a central role in the effector arm of Th2 immune responses elicited in allergic diseases and parasitic inflammation. Macrophage migration inhibitory factor (MIF), a proinflammatory cytokine that not only contributes to the immune response to infection but also promotes tissue damage in sterile inflammation, and infectious conditions, is important in Th2 immune responses. Activated Th2 cells have increased MIF mRNA and protein, while eosinophils have mRNA and the preformed protein and secrete high quantities of MIF upon stimulation. In animal models of eosinophilic inflammation such as asthma, rhinitis, dermatitis, eosinophilic esophagitis, and helminth infection, the blockage or the genetic lack of MIF causes a significant reduction of the cardinal signs observed in these diseases. Importantly, atopic patients have increased MIF in affected tissues. MIF also affects several aspects of eosinophil physiology including differentiation, survival, activation, and migration. In this chapter, we reviewed the current knowledge of the role of MIF in eosinophil biology and in eosinophilic inflammatory conditions.
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Contribution of MIF to the Pathogenesis of Allergic Diseases
1.1 Eosinophilic Inflammation
Eosinophils are differentiated bone marrow-produced inflammatory cells that transit the blood and are recruited to mucosal tissue, especially during the course of inflammatory conditions such as allergy and parasitic infections. IL-5 produced by Th2 cells, among other cells, is critical to eosinophil proliferation and terminal differentiation in the bone marrow, as well as their release in the blood. Along with IL-5, IL-3 and GM-CSF, may prime eosinophils to respond to chemoattractants such as eotaxin-1 (CCL11) produced at inflammatory sites [1]. Upon inflammation, eosinophils are stimulated and activated leading to selective secretion of a multitude of cytokines and chemokines, as well as lipid mediators and eosinophil cationic protein, a major basic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin [2, 3]. Although the effector activity of these cells is considered key to protect host tissue from parasites, the release of eosinophil granule contents may often contribute to tissue damage as well as remodeling in type-2 inflammatory responses [4].
1.2 The Role of MIF in Allergic Asthma and Rhinitis
The first observations suggesting that MIF could participate in allergic processes came from clinical studies with asthmatic and atopic dermatitis patients [5,6,7,8]. MIF concentrations are higher in the bronchoalveolar lavage (BAL) and sputum from asthmatic patients compared to healthy controls [7, 8]. Production and secretion of MIF by human eosinophils indicate that these cells are one of the several potential sources of MIF in asthmatic patients [7]. In fact, airway epithelial cells, macrophages, mast cells, and Th2 lymphocytes also produce and secrete MIF and likely contribute to the MIF produced during allergic episodes [9,10,11,12]. Overall, these studies added MIF to a long list of inflammatory mediators found in atopic patients with allergic asthma, which are produced by cells involved in the pathogenesis of this complex inflammatory condition. Moreover, polymorphisms in MIF gene have been associated with allergic diseases such as asthma and atopic dermatitis [13,14,15].
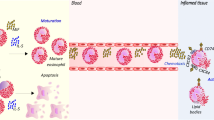
Studies using animal models of atopic asthma consistently demonstrated that MIF is increased in the BAL fluid and has a critical role in allergic inflammatory response, contributing to tissue eosinophilia, mucus production, and airway hyperreactivity (AHR) [12, 14, 16, 17]. MIF expression is also increased in the lungs in a mouse model of chronic asthma, both in epithelial cells and in infiltrating leukocytes [18]. The exact mechanism by which MIF promotes the pathogenesis of asthma is not completely understood. The lack of MIF has been shown to affect the allergic response in the models of ovalbumin-induced asthma in distinct ways (Fig. 1).
In some studies, the abrogation of the cardinal signs of asthma in the absence of MIF is associated with an impaired adaptive immune response, as demonstrated by reduced antigen-specific lymphocyte activation, Th2 cytokine production, and IgE concentrations [12, 14, 19]. Mizue and coworkers attributed the reduced activation of T lymphocytes and reduced production of Th2 cytokines to an impaired antigen presentation function of antigen-presenting cells obtained from T cell-depleted splenocytes from Mif −/− compared to wildtype (WT) mice [14]. Another study also demonstrating reduced antigen-specific T cell activation, Th2 cytokine production, and IgE concentrations in mice deficient in Mif indicates that macrophages and mast cells, but not DCs, from these animals are defective in activating antigen-specific CD4+ T lymphocytes [12]. Reconstitution of ovalbumin-sensitized Mif −/− mice with mast cells from WT mice restored the recruitment of eosinophils to the airways and the serum concentrations of IgE, indicating a role of mast cell-derived MIF in the priming phase of the adaptive immune response.
Using a model of epicutaneous sensitization with ovalbumin in the absence of adjuvant, Mif −/− mice have an impaired T cell response with reduced antigen-induced T cell proliferation, Th2 cytokine production, total IgE, and ovalbumin-specific IgG1 serum concentrations as well as lung eosinophilic inflammation upon intranasal challenge [19]. This study demonstrated that MIF contributes to both the sensitization and the elicitation phases of T cell activation. Interestingly, upon epicutaneous sensitization with ovalbumin, Mif −/− mice have an increased number of CD4+Foxp3+ T regulatory cells (Tregs) in draining lymph nodes [19]. Mice deficient in CD74, a MIF receptor, are also defective in generating a Th2 response to epicutaneous sensitization. Interestingly, MIF from T cells, but not from antigen-presenting cells, is essential for T cell activation. Mif −/− DO11.10 TCR transgenic T cells are defective in their proliferation and IL-2 production when compared with transgenic T cells derived from WT mice, independent of whether the antigen-presenting cells come from WT or Mif −/− mice [19].
A study using an experimental model of asthma induced by ovalbumin demonstrates that Mif −/− mice present a profound reduction of AHR, lung eosinophilia, mucus metaplasia lung inflammatory cytokines (IL-13, IL-5, and eotaxin), and lipid mediators (Cys-leukotrienes) despite high serum IgE and Th2 cytokine concentrations in draining lymph nodes [17]. Moreover, allergic WT mice show an increase of eosinophil numbers in the blood and bone marrow, which is not observed in the Mif −/− mice. Consistently, treatment of mice and rats with antiMIF antibodies in the challenge phase reduces AHR and tissue eosinophilia without affecting Th2 differentiation and IgE concentrations [16, 17, 20]. These features are also observed in transgenic mice over-expressing Thioredoxin-1 [21]. These transgenic mice compared to WT mice present reduced concentrations of MIF, IL-13, and eotaxin in the lungs, abrogation of the cardinal features of asthma but a preserved systemic Th2 response and IgE concentrations. Thioredoxin-1 binds MIF with high affinity and increases extracellular MIF internalization, suggesting that Thioredoxin-1 expressed on the cell surface serves as one of the MIF-binding molecules and inhibits MIF-mediated inflammatory signals [22]. Interestingly, MIF also belongs to the thioredoxin family of proteins, demonstrating thiol reductase activity [23,24,25].
In a model of allergic inflammation, MIF is also essential to lipid body biogenesis and leukotriene C4 synthesis in recruited eosinophils, demonstrated by a marked reduction in Mif −/− compared with WT mice [26]. Likewise, in vivo administration of recombinant MIF induces eosinophil recruitment to the site of installation and production of leukotriene C4 within newly formed lipid bodies, as demonstrated by EicosaCell methodology. Thus, the critical role of MIF in allergic asthma prevailed with or without impairment of the antigen-specific immune response. In fact, it is not clear why in some studies MIF is essential to Th2 differentiation, while in others it critically contributes to allergic inflammation without affecting the generation of antigen-specific Th2 lymphocytes or IgE concentrations. These observed differences are likely related to variations in the experimental protocols, genetic background, and housing conditions in the different studies.
Tissue remodeling is a characteristic of many chronic inflammatory diseases and an important feature of type-2-mediated pathologies. In asthmatic patients, the cumulative structural changes, including collagen deposition, increased thickness of the subepithelial reticular basement membrane, airway smooth muscle proliferation, goblet cell metaplasia, and mucus plugs, gradually cause progressive loss of pulmonary functions [27]. Although the mechanisms of tissue remodeling are not well defined and the participation of inflammation in this process is under dispute, eosinophils might contribute to tissue remodeling in some allergic conditions, including some groups of asthmatic individuals [27, 28]. Others, however, observed that eosinophils are dispensable for remodeling and inflammation in an experimental model of asthma induced by house dust mite (HDM) [29]. HDM is a complex and clinical relevant antigen associated with several aspects of asthma pathogenesis, including the activation of lung epithelial cells, dendritic cells, and basophils that culminate in the production of cytokines that affect innate lymphoid cells and T lymphocytes [30, 31].
Considering the abrogation of tissue eosinophilia in experimental models of asthma in the absence of MIF, it would be expected that in chronic asthma, tissue remodeling could be partially dependent on MIF. In fact, the use of a MIF small molecule antagonist, ISO-1, significantly reduces eosinophilic inflammation and prevents changes in airway remodeling in a mouse model of chronic asthma induced by long-term sensitization and challenged with ovalbumin [18]. The inhibitory effect of ISO-1 on airway remodeling is comparable to that of dexamethasone (DEX). The ability of ISO-1 to inhibit eosinophil infiltration and TGF-β expression in the lung tissue of ovalbumin-sensitized mice might have contributed to inhibition of airway remodeling [18]. Whether the observed effects are exclusively related to the blockage of MIF or to the effects of ISO-1 on another target requires further investigations.
In patients with atopic rhinitis, MIF expression is increased in biopsy specimens of the nasal mucous membrane, and markedly in infiltrating eosinophils [32]. Signs of allergic rhinitis induced by alum and ovalbumin immunization and ovalbumin intranasal challenge are abrogated in Mif −/− compared to WT mice [33]. These include a significant reduction of eosinophil infiltration and TNF expression in the nasal mucosa of Mif −/− mice.
1.3 The Role of MIF in Atopic Dermatitis
Atopic dermatitis is a chronic inflammatory condition that affects the skin, characterized by the presence of eczematous lesions and intense itching. It is a lifelong pathological condition that usually starts at early age, presents with acute flares and is associated with other atopic manifestations such as asthma, rhinitis, and food allergy [34]. Defects of the epidermal barrier with increased exposure to microbial and environmental stimuli are currently considered key to the pathogenesis. Atopic dermatitis is associated with a type-2 immune response with local infiltration of eosinophils and Th2 cells into skin lesions. These infiltrating Th2 lymphocytes are the main sources of IL-4, IL-5, and IL-13, which, together with eotaxin are considered to be important to the pathogenesis [34, 35].
Increased expression of MIF mRNA and protein is observed in inflammatory skin lesions and in sera from atopic dermatitis patients [6, 36]. Importantly, the serum concentrations of MIF in these patients decrease with clinical improvement, suggesting that MIF might be a marker of disease severity or a mediator that contributes to the inflammatory response and to the pathogenesis [5]. Studies using mouse models of atopic dermatitis indicate that MIF participates in the inflammatory response inducing Th2 cytokines and eotaxin in the skin, and promoting eosinophil recruitment [37]. Transgenic MIF mice have an increased Th2 response and tissue eosinophilia when sensitized and challenged with ovalbumin compared to WT mice. Conversely, the expression of inflammatory cytokines and skin eosinophilic infiltration observed in WT mice after repeated epicutaneous challenge with ovalbumin are virtually absent in Mif −/− mice [37]. Moreover, the use of a MIF-DNA vaccination protocol, that elicited the production of endogenous antiMIF antibodies, significantly reduced the inflammatory skin manifestations in mice sensitized and challenged with ovalbumin [38].
The use of more relevant allergens such as ragweed pollen or Japanese cedar pollen confirmed the critical role of MIF in atopic dermatitis, more specifically in a model of conjunctivitis [39]. In this model, mice are systemically immunized with pollen and alum and challenged with pollen via eye drops, after tape-striping the eyelid area. The numbers of conjunctiva- and eyelid-infiltrating eosinophils are significantly increased in pollen-sensitized MIF transgenic mice when compared with WT mice. This change correlates with increased mRNA expression of IL-5, IL-13, and eotaxin in the eyelid skin sites of MIF transgenic mice. Fibroblasts obtained from MIF transgenic mice have a significantly increased expression of eotaxin mRNA and protein upon stimulation with IL-4 compared to WT mice [39]. Conversely, fibroblasts from Mif −/− mice showed negligible expression of eotaxin upon stimulation with IL-4. Moreover, stimulation of mouse fibroblasts with recombinant MIF, IL-4, or IL-13 causes an increase in the expression of eotaxin in a mechanism dependent on CD74.
1.4 The Role of MIF in Eosinophilic Esophagitis
Eosinophilic esophagitis (EoE) is a chronic atopic disease, associated with a type-2 immune response, often triggered by environmental agents including food and aeroallergens. Esophageal eosinophilia is the main characteristic of human EoE, but the mechanisms involved in cell accumulation in the tissue and the exact role of eosinophils in disease pathogenesis are not fully understood. EoE patients have an increased deposition of subepithelial collagen fibers and thickening of the basal cell layer due to an increased proliferation of epithelial cells [40, 41].
In esophageal samples from patients with EoE, MIF expression correlates with the number of tissue eosinophils and is detected mostly within the cytosol of cells of the immune system, especially eosinophils [42]. MIF expression is remarkably high in biopsy samples from EoE, but an increase of MIF is also observed in Gastroesophageal Reflux Disease (GERD) patients relative to controls. The inflammatory response of EoE, based on a type-2 immune response, in contrast with the predominant type-1 response in GERD [43], creates an environment in which eosinophils are attracted to and persist within the inflamed mucosa.
In vitro MIF has the ability to directly promote eosinophil chemotaxis in a mechanism dependent on CXCR4 [42], a chemotactic receptor that interacts with SDF-1α/CXCL12 in addition to MIF [44, 45]. The results indicate that eosinophils constitute a major source of MIF at inflammatory sites in atopic diseases and that MIF influences eosinophil recruitment. Considering that MIF is present mostly in eosinophils within the esophageal mucosa, probably MIF is not a primary trigger in EoE but rather an inflammatory mediator in the effector phase of the disease.
Mif −/− mice are resistant to the increase in eosinophilic infiltration, collagen deposition, and IL-13 expression observed in WT mice in a model of allergic EoE [42]. In vivo administration of recombinant MIF increases tissue eosinophilia and collagen deposition in ovalbumin-sensitized mice. Treatment with antiMIF monoclonal antibody or with a CXCR4 antagonist (AMD3100) in the challenge phase is also highly effective in preventing the signs of EoE, further indicating that the axis MIF/CXCR4 is critically important in the effector phase of the allergic response. CXCR4 is expressed on eosinophils and basophils [46, 47], cells considered essential to the pathogenesis of EoE [48, 49]. Thus, it will be important to characterize the role of MIF and CXCR4 on basophil function and their specific role in the pathogenesis of EoE. Moreover, the contribution of SDF-1α/CXCL12, the cognate ligand of CXCR4, on EoE is unknown and deserves investigation. Together, these results suggest that targeting MIF or the CXCR4 receptor might constitute a therapeutic option to treat patients with EoE. Importantly, AMD3100 (Plerixafor) is approved by the FDA for short-term treatment, and a clinical trial using a long-term and low-dose protocol presented promising results [50].
2 MIF and Helminth Infestation
In past years, several studies analyzed the participation of MIF in helminth infection, pathological conditions frequently characterized by type-2 immune responses. Treatment of mice infected with Schistosoma japonicum with antiMIF has no effect in the area of the granuloma compared with IgG-treated controls [51]. Impairment of the Th2 polarization can inhibit eosinophilopoiesis, reduce the size of granulomas and fibrosis on S. mansoni infection, as observed in IL-5 −/− mice or in IL-13/IL4 double-deficient mice [52, 53]. In fact, these studies indicate a profibrotic role of IL-13. In infection with the murine cisticercosis helminthic parasite Taenia crassiceps, IgE concentrations are preserved in Mif −/− mice compared to WT mice, suggesting no impairement of the Th2 response in the absence of MIF [54]. S. mansoni-infected WT and Mif −/− mice have similar plasma concentrations of IL-5 and IL-13 and IgE concentration was even greater in infected Mif −/− mice compared to WT mice. Mif −/− mice infected with S. mansoni present greatly decreased egg granuloma sizes [55]. These granulomas contain fewer eosinophils, which is a phenomenon that is paralleled by decreased bone marrow eosinophilopoiesis in Mif −/− mice infected with S. mansoni. Together, these results indicate that in S. mansoni infection, MIF orchestrates bone marrow eosinophil differentiation and recruitment to granulomas. Although the area of granulomas is decreased in S. mansoni-infected Mif −/− mice, they present no decrease in fibrosis, the main cause of portal hypertension [55]. This result contrasts with reduced tissue remodeling in mice lacking MIF in models of allergic asthma and eosinophilic esophagitis, in which there is a significant reduction in IL-13 [18, 42].
In S. mansoni infection, similar numbers of adult forms and eggs are present in WT and Mif −/− mice [55]. Interestingly, eosinophils are efficient to kill the S. mansoni parasite in vitro [56] and to invade schistosomes in vivo [57], indicating that they might participate in defense mechanisms against the parasite. In vivo, however, two mouse lineages deficient in eosinophils have no gross alterations in worm burden or liver fibrosis upon S. mansoni infection [58]. In contrast, treatment of mice with neutralizing antibodies against MIF promotes an increased parasite burden and fertility on S. japonicum infection compared to control antibody-treated animals [51]. This effect is observed when treatment starts late after infection, but not before infection, suggesting that MIF promotes the control of infection during a determined time window. Moreover, Mif −/− mice have previously been shown to poorly control infection with T. crassiceps [54]. A recent study has shown that MIF deficiency in a murine infection with Nippostrongylus brasiliensis, a helminth similar to the human hookworm, reduced the intestine parasite burden and increased the Th2 response specifically in the draining lymph nodes but not in the spleens [59]. Enhanced Th2 response in this scenario was directly related to a decreased activation of NF-κB and consequent reduction in IL-6 expression in T CD4 Mif −/− cells. The MIF tautomerase inhibitor, sulforaphane, used in WT mice infected with N. brasiliensis also led to an enhanced Th2 response and clearance of parasite burden, suggesting that MIF’s enzymatic activity might have a role in helminth infestation [59]. It is possible that the different outcomes of helminthic infections in the absence of MIF reflect the susceptibility of these parasites to the different defense mechanisms in which MIF interferes, which are currently poorly established.
3 MIF Orthologues from Helminths
Several parasites have orthologues of vertebrate MIF that are likely involved in escape mechanisms [60]. Brugia malayi-MIF is the first identified cytokine orthologue from helminth parasites that has the ability to modify host immune responses promoting parasite survival. In fact, MIF orthologues from Brugia malayi induce eosinophil inflammation and affect other parameters of the inflammatory response dependent on macrophage activation [61, 62]. Moreover, in association with IL-4, Brugia malayi-MIF causes the induction of alternatively activated macrophages [63]. Exposure of monocytes to a MIF orthologue from Strongiloidis ratti causes the release of IL-10 instead of TNF, suggesting the involvement of the secreted parasite MIF in immune evasion mechanisms [64]. A MIF orthologue from the nematode Anisakis simplex also causes an increase in IL-10 production. This effect is associated with an increased recruitment of CD4+CD25+Foxp3+ regulatory T cells (Tregs) and inhibition of allergic airway inflammation, indicating an opposite role of this MIF in asthma [65]. The induction of IL-10 and Tregs by the MIF orthologue is dependent on TLR2 [66, 67].
4 MIF in Eosinophil Biology
4.1 MIF Is Produced by Eosinophils
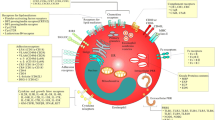
Eosinophils contribute to tissue damage and tissue remodeling in type-2 inflammatory responses. Previous studies demonstrated that MIF is produced by a variety of cells and eosinophils constitute an important source of MIF in allergic inflammation and helminth infection [68,69,70]. Unstimulated human eosinophils have preformed MIF protein and secrete high quantities of MIF upon stimulation with the inflammatory mediators C5a or IL-5 [69]. Similarly, stimulation with PMA causes an early and sustained secretion of MIF in a PKC-dependent manner. At later time points, the production of MIF requires neosynthesis as indicated by reduced secretion in cyclohexamide-treated eosinophils. In vivo, eosinophils present in the inflamed tissue of patients with eosinophilic esophagitis have preformed MIF in the cytosol, constituting the main cell population expressing MIF in this disease [42]. A number of studies have shown important effects of MIF on eosinophil biology (Fig. 2).
4.2 MIF Promotes Eosinophil Recruitment and Chemotaxis
MIF has the ability to directly promote human eosinophil chemotaxis and this effect occurs in a similar concentration range as that of eotaxin [42]. Both, ISO-1, a MIF antagonist, and AMD3100, a CXCR4 antagonist, significantly inhibit the chemotactic effect of MIF. Similarly, MIF promotes the chemotaxis of mouse eosinophils dependent on CXCR2 and CXCR4 [55, 71]. Intrapleural administration of recombinant MIF in mice induces the recruitment of eosinophils [71]. Similarly, intranasal administration of MIF to mice increases the number of eosinophils in the esophagus [42]. As previously discussed, mice lacking MIF and the blockage of MIF or its receptors (eg. CD74, CXCR4) cause a profound reduction of eosinophil numbers in inflamed tissues in experimental models of allergic and parasitic diseases, demonstrating the general importance of MIF in eosinophil recruitment.
4.3 MIF Causes the Formation of Lipid Bodies and Production of Inflammatory Mediators by Eosinophils
Stimulation of human eosinophils with recombinant MIF induces an increase in the numbers of cytoplasmic lipid bodies and enhanced production of eotaxin and leukotriene C4 [71]. The generation of lipid bodies by MIF is dependent on CD74 expressed on eosinophils. MIF-induced eotaxin acts in an autocrine/paracrine fashion contributing to the generation of lipid bodies dependent on CCR3. Conversely, stimulation of human eosinophils with eotaxin causes the generation of lipid bodies, an effect reverted by antiCD74 neutralizing antibody. Similarly, treatment with eotaxin causes a reduced generation of lipid bodies in eosinophils from Mif −/− mice compared to eosinophils from WT. Suboptimal concentrations of eotaxin and MIF have a synergistic effect to induce the generation of lipid bodies [71]. Together, these results suggest that a crosstalk between MIF and eotaxin causing activation of eosinophils.
4.4 MIF Contributes to Eosinophil Maturation Induced by IL-5
IL-5 is a cytokine critically involved in the terminal differentiation of committed eosinophil precursors [72]. Using an in vitro system of eosinophil maturation induced by IL-5, bone marrow from Mif −/− mice has a profound defect in generating eosinophils even in the presence of high IL-5 concentrations [55]. Inclusion of rMIF in the Mif −/− cultures fully restores the ability of IL-5 to promote accumulation of eosinophils to the numbers achieved in WT. Treatment of Mif −/− cell cultures with the pan-caspase inhibitor zVAD restores the number of eosinophils accumulated in the presence of IL-5 to the number of WT controls, indicating that in the absence of MIF the eosinophil precursors are more prone to die by apoptosis [55]. However, in mature eosinophils, the antiapoptotic effect of MIF is minor when compared with GM-CSF [73]. These results indicate that MIF acts as a cofactor necessary to allow optimal IL-5-driven eosinophilopoiesis through the protection of eosinophils, during terminal differentiation induced by IL-5, from programmed cell death. These results also suggest that reduced blood and tissue eosinophilia of Mif −/− mice upon type-2 immune responses could in part be due to a defect in eosinophilopoiesis.
5 Perspectives
In recent years several important discoveries regarding the intricate mechanisms of allergic inflammation, especially asthma pathogenesis, have been made with the use of complex and clinically relevant antigens such as house dust mite. It became clear that lung epithelial cells triggering the immune response to allergens by secreting IL-33, IL-25, and TSLP are important. These cytokines contribute both directly and indirectly to the recruitment and activation of type-2 innate lymphoid and Th2 cells, also critically involved in asthma pathogenesis. In order to further establish the role of MIF in the physiopathology of asthma, it will be essential to use better experimental models, including the use of house dust mite. Moreover, future studies should address the role of different MIF cell sources and targets of MIF action with the use of mouse strains with cell-specific deletion of MIF and its receptors. Finally, the use of antiMIF neutralizing antibodies, MIF antagonists, and CXCR4 antagonist in clinical trials of asthma and other allergic diseases will define the importance of MIF in the pathogenesis of human allergy and the putative beneficial effects of blocking MIF in these conditions.
References
Sanderson CJ (1992) Interleukin-5, eosinophils, and disease. Blood 79(12):3101–3109
Spencer LA et al (2014) Eosinophil secretion of granule-derived cytokines. Front Immunol 5:496
Neves JS, Weller PF (2009) Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol 21(6):694–699
Williams TJ (2004) The eosinophil enigma. J Clin Invest 113(4):507–509
Shimizu T et al (1997) Macrophage migration inhibitory factor is an essential immunoregulatory cytokine in atopic dermatitis. Biochem Biophys Res Commun 240(1):173–178
Shimizu T et al (1999) Increased production of macrophage migration inhibitory factor by PBMCs of atopic dermatitis. J Allergy Clin Immunol 104(3 Pt 1):659–664
Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, Bucala R, Donnelly SC (1998) Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Investig 101:2869–2874
Yamaguchi E et al (2000) Macrophage migration inhibitory factor (MIF) in bronchial asthma. Clin Exp Allergy 30(9):1244–1249
Calandra T et al (1994) The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 179(6):1895–1902
Bacher M et al (1996) An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A 93(15):7849–7854
Makita H et al (1998) Effect of anti-macrophage migration inhibitory factor antibody on lipopolysaccharide-induced pulmonary neutrophil accumulation. Am J Respir Crit Care Med 158(2):573–579
Wang B et al (2006) Cutting edge: deficiency of macrophage migration inhibitory factor impairs murine airway allergic responses. J Immunol 177(9):5779–5784
Hizawa N et al (2004) Functional polymorphisms in the promoter region of macrophage migration inhibitory factor and atopy. Am J Respir Crit Care Med 169(9):1014–1018
Mizue Y et al (2005) Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A 102(40):14410–14415
Wu J et al (2009) Association of MIF promoter polymorphisms with childhood asthma in a northeastern Chinese population. Tissue Antigens 73(4):302–306
Kobayashi M et al (2006) Role of macrophage migration inhibitory factor in ovalbumin-induced airway inflammation in rats. Eur Respir J 27(4):726–734
Magalhaes ES et al (2007) Macrophage migration inhibitory factor is essential for allergic asthma but not for Th2 differentiation. Eur J Immunol 37(4):1097–1106
Chen PF et al (2010) ISO-1, a macrophage migration inhibitory factor antagonist, inhibits airway remodeling in a murine model of chronic asthma. Mol Med 16(9–10):400–408
Das R et al (2011) Role of macrophage migration inhibitory factor in the Th2 immune response to epicutaneous sensitization. J Clin Immunol 31(4):666–680
Amano T, Nishihira J, Miki I (2007) Blockade of macrophage migration inhibitory factor (MIF) prevents the antigen-induced response in a murine model of allergic airway inflammation. Inflamm Res 56(1):24–31
Torii M et al (2010) Thioredoxin suppresses airway inflammation independently of systemic Th1/Th2 immune modulation. Eur J Immunol 40(3):787–796
Son A et al (2009) Direct association of thioredoxin-1 (TRX) with macrophage migration inhibitory factor (MIF): regulatory role of TRX on MIF internalization and signaling. Antioxid Redox Signal 11(10):2595–2605
Kleemann R et al (1999) Characterization of catalytic centre mutants of macrophage migration inhibitory factor (MIF) and comparison to Cys81Ser MIF. Eur J Biochem 261(3):753–766
Potolicchio I, Santambrogio L, Strominger JL (2003) Molecular interaction and enzymatic activity of macrophage migration inhibitory factor with immunorelevant peptides. J Biol Chem 278(33):30889–30895
Thiele M, Bernhagen J (2005) Link between macrophage migration inhibitory factor and cellular redox regulation. Antioxid Redox Signal 7(9–10):1234–1248
Vieira-de-Abreu A1, Calheiros AS, Mesquita-Santos FP, Magalhães ES, Mourão-Sá D, Castro-Faria-Neto HC, Bozza MT, Bandeira-Melo C, Bozza PT (2011) Crosstalk between macrophage migration inhibitory factor and eotaxin in allergic eosinophil activation forms leukotriene C4-synthesizing lipid bodies. Am J Respir Cell Mol Biol 44(4):509–16. doi: 10.1165/rcmb.2010-0004OC. Epub 2010 Jun 10
Saglani S, Lloyd CM (2015) Novel concepts in airway inflammation and remodelling in asthma. Eur Respir J 46(6):1796–1804
Humbles AA et al (2004) A critical role for eosinophils in allergic airways remodeling. Science 305(5691):1776–1779
Fattouh R et al (2011) Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. Am J Respir Crit Care Med 183(2):179–188
Gregory LG, Lloyd CM (2011) Orchestrating house dust mite-associated allergy in the lung. Trends Immunol 32(9):402–411
Lambrecht BN, Hammad H (2015) The immunology of asthma. Nat Immunol 16(1):45–56
Nakamaru Y et al (2004) Macrophage migration inhibitory factor in allergic rhinitis: its identification in eosinophils at the site of inflammation. Ann Otol Rhinol Laryngol 113(3 Pt 1):205–209
Nakamaru Y et al (2005) Macrophage migration inhibitory factor (MIF) contributes to the development of allergic rhinitis. Cytokine 31(2):103–108
Weidinger S, Novak N (2016) Atopic dermatitis. Lancet 387(10023):1109–1122
Yawalkar N et al (1999) Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J Invest Dermatol 113(1):43–48
Shimizu T (2005) Role of macrophage migration inhibitory factor (MIF) in the skin. J Dermatol Sci 37(2):65–73
Yoshihisa Y et al (2011) Macrophage migration inhibitory factor is essential for eosinophil recruitment in allergen-induced skin inflammation. J Invest Dermatol 131(4):925–931
Hamasaka A et al (2009) DNA vaccination against macrophage migration inhibitory factor improves atopic dermatitis in murine models. J Allergy Clin Immunol 124(1):90–99
Nagata Y et al (2015) Role of macrophage migration inhibitory factor (MIF) in pollen-induced allergic conjunctivitis and pollen dermatitis in mice. PLoS One 10(2):e0115593
Chehade M et al (2007) Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 45(3):319–328
Li-Kim-Moy JP et al (2011) Esophageal subepithelial fibrosis and hyalinization are features of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 52(2):147–153
de Souza HS et al (2015) Macrophage migration inhibitory factor promotes eosinophil accumulation and tissue remodeling in eosinophilic esophagitis. Mucosal Immunol 8(5):1154–1165
Rieder F et al (2007) Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology 132(1):154–165
Bernhagen J et al (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 13(5):587–596
Pawig L et al (2015) Diversity and inter-connections in the CXCR4 chemokine receptor/ligand family: molecular perspectives. Front Immunol 6:429
Nagase H et al (2000) Expression of CXCR4 in eosinophils: functional analyses and cytokine-mediated regulation. J Immunol 164(11):5935–5943
Iikura M et al (2001) Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukoc Biol 70(1):113–120
Blanchard C, Rothenberg ME (2008) Basic pathogenesis of eosinophilic esophagitis. Gastrointest Endosc Clin N Am 18(1):133–143; x
Noti M et al (2013) Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med 19(8):1005–1013
McDermott DH et al (2014) A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood 123(15):2308–2316
Stavitsky AB et al (2003) Blockade of macrophage migration inhibitory factor (MIF) in Schistosoma japonicum-infected mice results in an increased adult worm burden and reduced fecundity. Parasite Immunol 25(7):369–374
Reiman RM et al (2006) Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun 74(3):1471–1479
Fallon PG et al (2000) Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol 164(5):2585–2591
Rodriguez-Sosa M et al (2003) Macrophage migration inhibitory factor plays a critical role in mediating protection against the helminth parasite Taenia crassiceps. Infect Immun 71(3):1247–1254
Magalhaes ES et al (2009) Macrophage migration inhibitory factor is critical to interleukin-5-driven eosinophilopoiesis and tissue eosinophilia triggered by Schistosoma mansoni infection. FASEB J 23(4):1262–1271
David JR, Butterworth AE, Vadas MA (1980) Mechanism of the interaction mediating killing of Schistosoma mansoni by human eosinophils. Am J Trop Med Hyg 29(5):842–848
Davies SJ et al (2005) In vivo imaging of tissue eosinophilia and eosinopoietic responses to schistosome worms and eggs. Int J Parasitol 35(8):851–859
Swartz JM et al (2006) Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood 108(7):2420–2427
Damle SR et al (2017) Macrophage migration inhibitory factor deficiency enhances immune response to Nippostrongylus brasiliensis. Mucosal Immunol 10(1):205–214
Pastrana DV et al (1998) Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun 66(12):5955–5963
Falcone FH et al (2001) A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J Immunol 167(9):5348–5354
Zang X et al (2002) Homologues of human macrophage migration inhibitory factor from a parasitic nematode. Gene cloning, protein activity, and crystal structure. J Biol Chem 277(46):44261–44267
Prieto-Lafuente L et al (2009) MIF homologues from a filarial nematode parasite synergize with IL-4 to induce alternative activation of host macrophages. J Leukoc Biol 85(5):844–854
Younis AE et al (2012) Characterization of a secreted macrophage migration inhibitory factor homologue of the parasitic nematode Strongyloides acting at the parasite-host cell interface. Microbes Infect 14(3):279–289
Park SK et al (2009) Macrophage migration inhibitory factor homologs of anisakis simplex suppress Th2 response in allergic airway inflammation model via CD4+CD25+Foxp3+ T cell recruitment. J Immunol 182(11):6907–6914
Cho MK, Lee CH, Yu HS (2011) Amelioration of intestinal colitis by macrophage migration inhibitory factor isolated from intestinal parasites through toll-like receptor 2. Parasite Immunol 33(5):265–275
Cho MK et al (2015) TLR2-dependent amelioration of allergic airway inflammation by parasitic nematode type II MIF in mice. Parasite Immunol 37(4):180–191
Calandra T, Roger T (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3(10):791–800
Rossi RE, Monasterolo G, Operti D (1998) A comparative study of the tryptase release test and the cellular allergen stimulation test (CAST) in mite sensitive patients. Clin Exp Allergy 28(6):752–757
Souza AL et al (2005) Potential role of the chemokine macrophage inflammatory protein 1alpha in human and experimental schistosomiasis. Infect Immun 73(4):2515–2523
Vieira-de-Abreu A et al (2011) Cross-talk between macrophage migration inhibitory factor and eotaxin in allergic eosinophil activation forms leukotriene C(4)-synthesizing lipid bodies. Am J Respir Cell Mol Biol 44(4):509–516
Rothenberg ME, Hogan SP (2006) The eosinophil. Annu Rev Immunol 24:147–174
Baumann R et al (2003) Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria-dependent death pathway. FASEB J 17(15):2221–2230
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bozza, M.T., Paiva, C.N., Olsen, P.C. (2017). MIF in Eosinophilic Inflammation. In: Bucala, R., Bernhagen, J. (eds) MIF Family Cytokines in Innate Immunity and Homeostasis. Progress in Inflammation Research. Springer, Cham. https://doi.org/10.1007/978-3-319-52354-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-52354-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52352-1
Online ISBN: 978-3-319-52354-5
eBook Packages: MedicineMedicine (R0)